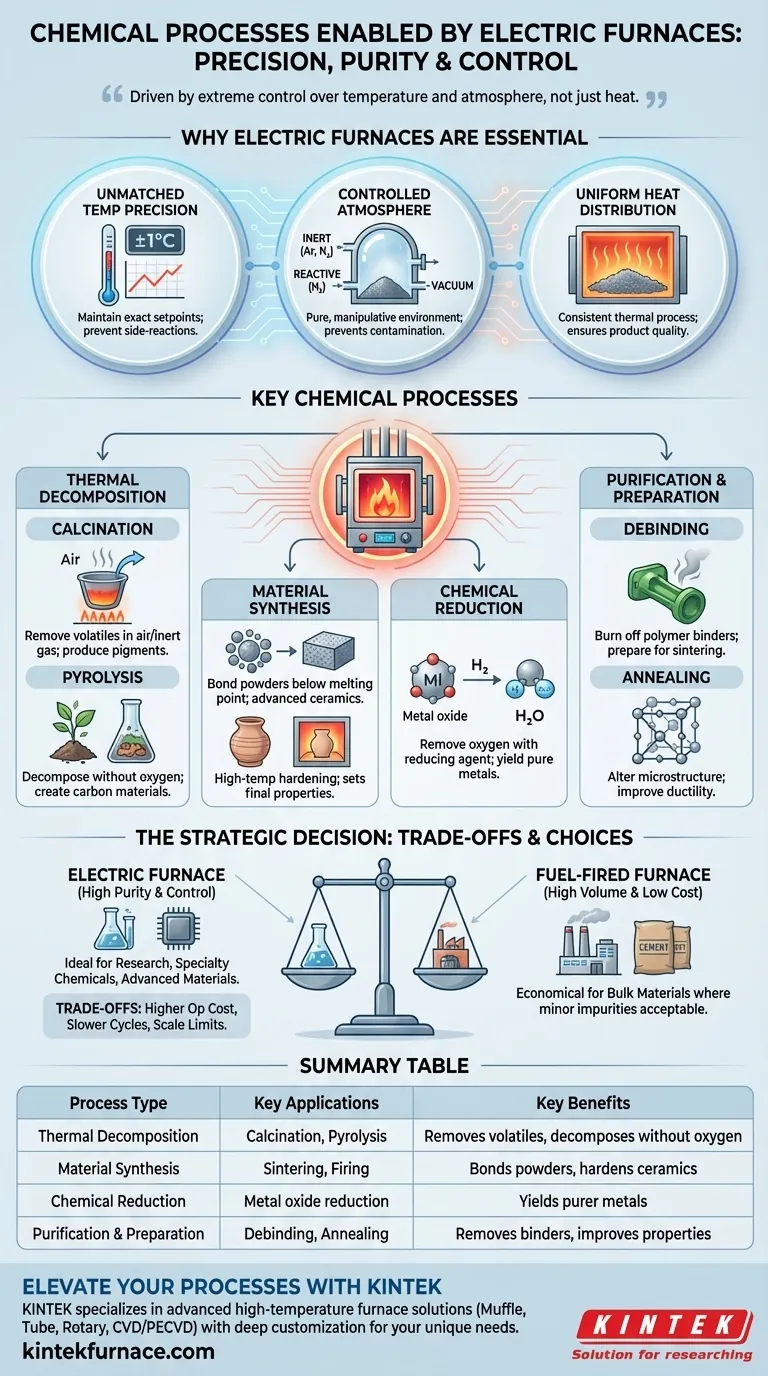
At their core, electric furnaces are used for chemical processes that demand extremely precise temperature control and specific atmospheric conditions. The most common applications include calcination for material purification, pyrolysis for thermal decomposition without oxygen, and sintering to create advanced ceramics and powdered metal parts.
The decision to use an electric furnace is not about heat alone; it's about control. These furnaces are chosen when the chemical integrity and final properties of a material are so sensitive that the impurities and temperature fluctuations of a fuel-fired furnace are unacceptable.

Why Electric Furnaces Are Essential in Chemical Processing
Unlike furnaces that burn fuel, electric furnaces generate heat via electrical resistance. This fundamental difference provides three critical advantages for sensitive chemical work.
Unmatched Temperature Precision
Electric heating elements, governed by modern controllers, can maintain a setpoint with incredible accuracy, often within a single degree. This precision is vital for triggering specific chemical reactions while preventing unwanted side-reactions that can occur at slightly higher or lower temperatures.
Controlled Atmospheric Conditions
Because there is no combustion, the atmosphere inside an electric furnace is pure and easily manipulated. The sealed chamber can be filled with an inert gas (like argon or nitrogen) to prevent oxidation, a reactive gas (like hydrogen) for chemical reduction, or even a vacuum. This protects the material from contamination.
Uniform Heat Distribution
Well-designed electric furnaces provide exceptionally uniform heat throughout the chamber. This ensures that every part of the material batch undergoes the same thermal process, leading to consistent product quality, which is paramount in the production of high-value specialty chemicals.
Key Chemical Processes Enabled by Electric Furnaces
The control offered by electric furnaces makes them the go-to solution for several classes of high-temperature chemical transformations.
Thermal Decomposition (Calcination & Pyrolysis)
Calcination is the process of heating a solid to high temperatures in the presence of air or an inert gas to remove volatile components or trigger a phase transition. A common example is producing pigments or catalysts.
Pyrolysis is a similar process but is performed in a complete absence of oxygen. This thermally decomposes organic materials without burning them, a key step in creating certain types of carbon materials or advanced chemicals.
Material Synthesis (Sintering & Firing)
Sintering involves heating compacted powders to just below their melting point. This causes the individual particles to bond and fuse, creating a solid, dense object. It is fundamental to producing high-performance ceramics, metal-injection-molded (MIM) parts, and cermets.
Firing is a broader term, often used in ceramics, to describe the high-temperature heating that permanently hardens the material and sets its chemical and physical properties.
Chemical Reduction
Certain processes require removing oxygen from a compound, typically a metal oxide. An electric furnace can be filled with a reducing atmosphere, like hydrogen gas, to strip oxygen atoms from the material at high temperatures, yielding a purer form of the metal.
Purification and Preparation (Debinding & Annealing)
Debinding is a critical preparatory step before sintering. It involves slowly heating a "green" part to burn off the polymer binders that were used to hold the powdered material in shape during molding.
Annealing is a heat treatment that alters a material's microstructure to improve its ductility and reduce hardness. While often considered a metallurgical process, the underlying changes are driven by solid-state chemical diffusion and crystal rearrangement.
Understanding the Trade-offs
While powerful, electric furnaces are not the universal solution. Their selection involves clear trade-offs against traditional fuel-fired alternatives.
Higher Operational Cost
Electricity is generally a more expensive energy source per unit of heat (BTU) than natural gas or coal. For bulk processes where raw cost is the primary driver, fuel-fired furnaces are often more economical.
Slower Heating Cycles
The heavy insulation required for efficiency gives many electric furnaces significant thermal mass. This can result in longer ramp-up and cool-down times compared to direct-fired systems, potentially impacting overall throughput.
Scale and Throughput Limitations
For specialty chemical and lab applications, electric furnaces are ideal. However, for producing massive quantities of bulk materials like cement or lime, large, continuous rotary kilns powered by fuel are far more common and cost-effective.
Making the Right Choice for Your Goal
Your choice of heating technology should be dictated by the specific requirements of your chemical process and business goals.
- If your primary focus is high purity and process control: An electric furnace is the definitive choice for research, specialty chemicals, and advanced materials where contamination is not an option.
- If your primary focus is high-volume, low-cost production: A fuel-fired furnace is likely the more economical solution for bulk materials where minor impurities from combustion are acceptable.
- If your primary focus is creating a specific, non-oxidizing atmosphere: The sealed and non-combustion environment of an electric furnace is a necessity for processes requiring inert or reducing conditions.
Ultimately, selecting an electric furnace is a strategic decision to prioritize the precision and purity of your final product.
Summary Table:
| Process Type | Key Applications | Key Benefits |
|---|---|---|
| Thermal Decomposition | Calcination, Pyrolysis | Removes volatiles, decomposes without oxygen |
| Material Synthesis | Sintering, Firing | Bonds powders, hardens ceramics |
| Chemical Reduction | Metal oxide reduction | Yields purer metals with reducing atmospheres |
| Purification and Preparation | Debinding, Annealing | Removes binders, improves material properties |
Ready to elevate your chemical processes with precision and purity? KINTEK specializes in advanced high-temperature furnace solutions tailored for diverse laboratories. Leveraging exceptional R&D and in-house manufacturing, we offer products like Muffle, Tube, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems, all backed by strong deep customization to meet your unique experimental needs. Contact us today to discuss how our furnaces can enhance your results and efficiency!
Visual Guide
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Split Multi Heating Zone Rotary Tube Furnace Rotating Tube Furnace
- Laboratory Quartz Tube Furnace RTP Heating Tubular Furnace
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
People Also Ask
- What is an example of a material prepared using a tube furnace? Master Precise Material Synthesis
- What safety measures are essential when operating a lab tube furnace? A Guide to Preventing Accidents
- Why is a tube furnace utilized for the heat treatment of S/C composite cathode materials? Optimize Battery Stability
- How is a Vertical Tube Furnace used for fuel dust ignition studies? Model Industrial Combustion with Precision
- What recent improvements have been made to lab tube furnaces? Unlock Precision, Automation & Safety



















