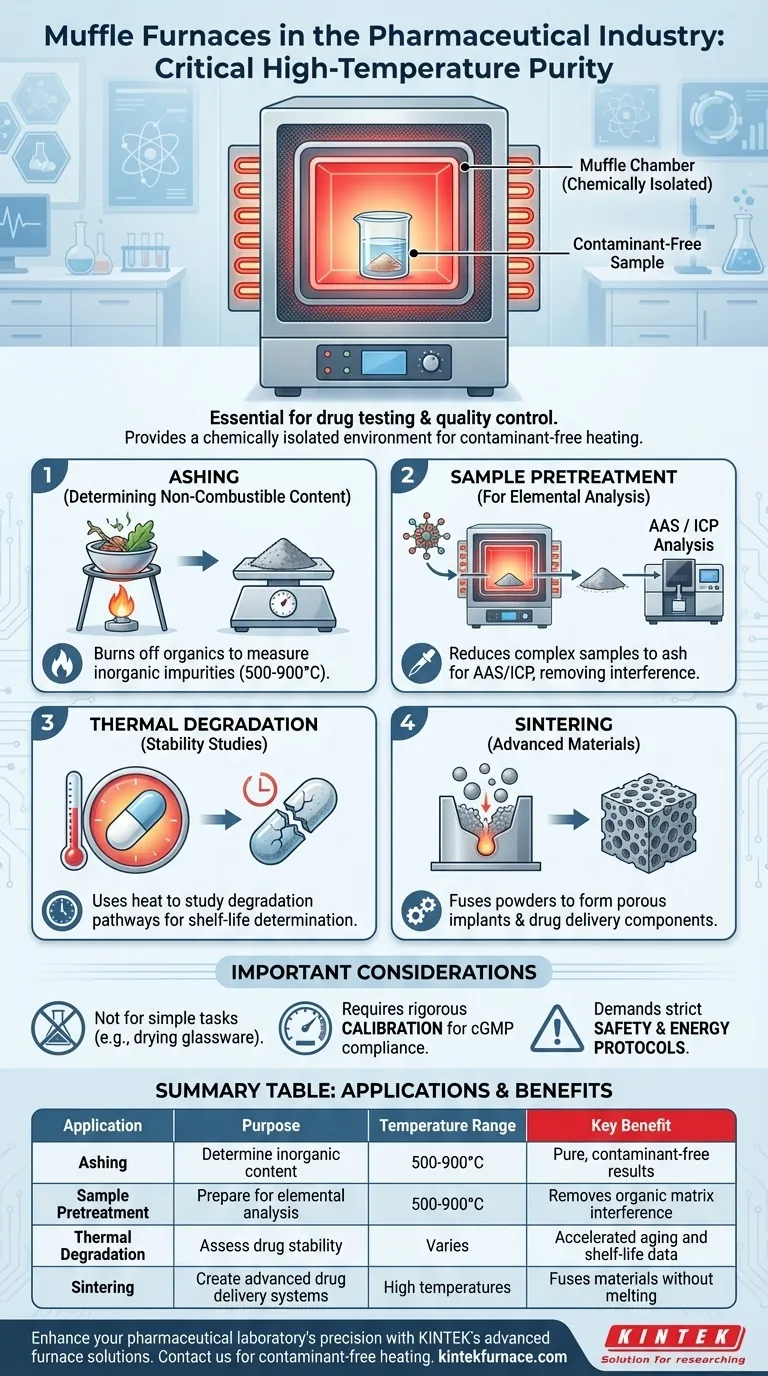
In the pharmaceutical industry, a muffle furnace serves as a critical tool for high-temperature analysis and material processing. It is primarily used for drug testing, the preparation of medical samples for further analysis, and quality control procedures that demand a precisely controlled, contaminant-free heating environment. Key applications include determining the inorganic content of a substance and sintering materials for advanced drug delivery systems.
The core value of a muffle furnace is not just its high heat, but its ability to provide a chemically isolated environment. By separating the sample from the heating elements, it ensures that analytical results, such as ash content, are pure, repeatable, and free from external contamination—an absolute requirement in pharmaceutical quality assurance.

The Core Function: High-Temperature Purity
A muffle furnace's design is central to its role in sensitive industries like pharmaceuticals. Understanding its function reveals why it is the standard for specific analytical tasks.
What is a Muffle Furnace?
A muffle furnace contains an insulated outer chamber that houses heating elements. Inside, a separate, sealed chamber—the "muffle"—holds the sample.
This design is critical. The muffle isolates the sample from any gases or impurities produced by the heating elements during combustion, ensuring the heating process itself does not contaminate the analysis.
Why Purity is Non-Negotiable in Pharma
Pharmaceutical analysis demands extreme accuracy. Even trace amounts of contamination can invalidate test results, compromise patient safety, and lead to regulatory failure.
The muffle furnace provides the controlled, inert, high-temperature environment necessary to meet these stringent standards for quality control and research.
Key Pharmaceutical Applications
The unique capabilities of a muffle furnace support several essential processes in both pharmaceutical research and manufacturing.
Determining Non-Combustible Content (Ashing)
The most common application is ashing. This process involves heating a sample to a high temperature (often 500-900°C) to completely burn off all organic and volatile substances.
What remains is only the inorganic, non-combustible material, or ash. Analyzing this ash is a fundamental quality control test to measure the total amount of inorganic impurities or mineral content in a drug substance or raw material.
Sample Pretreatment for Elemental Analysis
Ashing is a form of sample pretreatment. By reducing a complex organic sample to a simple inorganic ash, the furnace prepares it for further, more sophisticated analysis.
Techniques like Atomic Absorption Spectroscopy (AAS) or Inductively Coupled Plasma (ICP) can then accurately identify and quantify the specific elemental composition of the ash without interference from the original organic matrix.
Thermal Degradation and Stability Studies
Muffle furnaces are used to conduct accelerated aging and stress testing. By subjecting a drug product to extreme heat, researchers can study its thermal degradation pathways.
This data is vital for determining a product's shelf-life, understanding its stability under various conditions, and ensuring it remains safe and effective throughout its lifecycle.
Sintering for Advanced Materials
In pharmaceutical development, sintering uses high heat to fuse powdered materials into a solid mass without melting them.
This process, which requires the uniform temperature control of a muffle furnace, is used to create porous ceramic implants or components for specialized drug delivery systems that release medication over time.
Understanding the Trade-offs and Considerations
While powerful, a muffle furnace is a specialized instrument. Its application must be appropriate for the goal.
Not for All Heating Tasks
A muffle furnace is overkill for simple processes like drying glassware or low-temperature incubation. Its value lies in high-temperature applications where sample isolation and purity are paramount.
The Importance of Calibration
For use in regulated pharmaceutical environments (cGMP), the furnace's temperature controller must be rigorously calibrated and validated. Proving temperature accuracy and uniformity within the chamber is essential for compliant and reliable results.
Safety and Energy Protocols
Operating at extremely high temperatures, these furnaces consume significant energy and require strict safety protocols. Proper training, personal protective equipment (PPE), and placement in a well-ventilated area with fire-resistant surfaces are mandatory.
Making the Right Choice for Your Goal
To apply this tool effectively, align its function with your specific objective.
- If your primary focus is Quality Control (QC): Use the muffle furnace for ashing to quantify the inorganic impurity profile of raw materials and finished drug products.
- If your primary focus is Research & Development (R&D): Leverage the furnace for thermal stability studies and for sintering novel materials in the creation of advanced drug delivery systems.
- If your primary focus is Analytical Chemistry: Employ the furnace as the definitive first step in sample preparation for any elemental analysis requiring the complete removal of the organic matrix.
Mastering the muffle furnace is about ensuring the absolute purity and integrity of your analytical results—a cornerstone of modern pharmaceutical science.
Summary Table:
| Application | Purpose | Temperature Range | Key Benefit |
|---|---|---|---|
| Ashing | Determine inorganic content | 500-900°C | Pure, contaminant-free results |
| Sample Pretreatment | Prepare for elemental analysis | 500-900°C | Removes organic matrix interference |
| Thermal Degradation Studies | Assess drug stability | Varies | Accelerated aging and shelf-life data |
| Sintering | Create advanced drug delivery systems | High temperatures | Fuses materials without melting |
Enhance your pharmaceutical laboratory's precision and compliance with KINTEK's advanced high-temperature furnace solutions. Leveraging exceptional R&D and in-house manufacturing, we offer Muffle, Tube, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems, all with deep customization to meet your unique experimental needs. Ensure contaminant-free heating and reliable results—contact us today to discuss how our furnaces can support your QC, R&D, and analytical goals!
Visual Guide
Related Products
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- Multi Zone Laboratory Quartz Tube Furnace Tubular Furnace
People Also Ask
- What role does a muffle furnace play in the conversion of S-1@TiO2? Achieve Precision Calcination of Nanospheres
- What is the primary use of a muffle furnace in the assembly of side-heated resistive gas sensors? Expert Annealing Guide
- How does a muffle furnace contribute to kaolin-modified biochar? Optimize Pyrolysis & Mineral Integration
- What role does a muffle furnace play in g-C3N4 synthesis? Mastering Thermal Polycondensation for Semiconductors
- Why are precision stirring and drying equipment necessary for photocatalytic materials? Master Microstructure Control



















