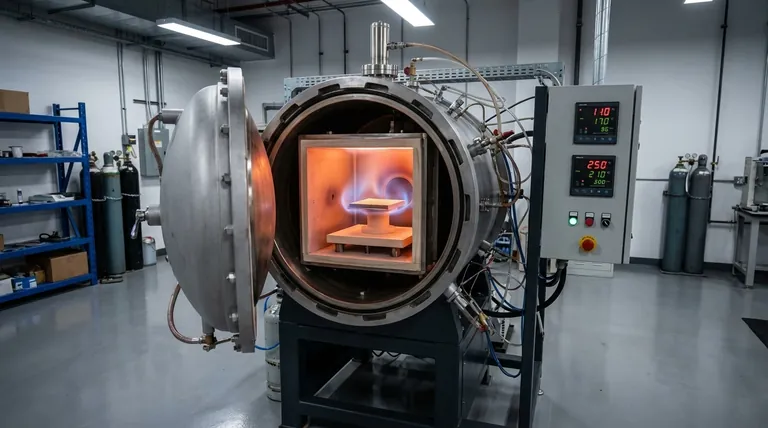
Furnace cooling protects CoCrFeNi(Cu) coatings by strictly controlling the thermal gradient and atmospheric exposure immediately following the sintering process. This method allows the sample to decrease in temperature slowly within a vacuum, mitigating the thermal shock that typically leads to catastrophic structural failure.
The primary function of furnace cooling is to prevent the accumulation of residual thermal stresses caused by mismatching expansion coefficients, ensuring the coating remains crack-free and metallurgically bonded to the substrate.
The Mechanics of Stress Mitigation
Managing Thermal Expansion Mismatch
During high-temperature sintering, both the coating and the substrate expand. However, they rarely expand and contract at the exact same rate due to differences in their coefficients of thermal expansion (CTE).
If the assembly is cooled too quickly, one material will contract faster than the other. This rapid differential contraction generates immense tension at the interface, threatening the bond formed during the hot pressing phase.
Preventing Structural Failure
Furnace cooling extends the cooling timeline, allowing the thermal energy to dissipate gradually.
This controlled pace permits the coating and substrate to contract in unison, or allows time for atomic-level stress relaxation mechanisms to activate. This directly prevents the formation of macro-cracks within the coating and prevents the coating from peeling (delamination) off the substrate.
Preserving Alloy Chemistry
Shielding Reactive Elements
The "vacuum" component of the furnace cooling process is just as critical as the temperature control. Elements within the CoCrFeNi high-entropy alloy—specifically Chromium, Iron, and Nickel—are highly reactive to oxygen at elevated temperatures.
Even after the active sintering phase is complete, the coating remains vulnerable to oxidation until it cools significantly. Maintaining the vacuum throughout the cooling phase prevents oxygen from attacking the surface.
Ensuring Material Purity
By maintaining a high vacuum (e.g., 2 Pa) until the sample reaches a safe temperature, the process prevents the formation of oxide inclusions.
These inclusions act as defects that degrade mechanical properties. Furthermore, the vacuum environment continues to remove adsorbed gases from the surface, ensuring the final coating retains superior corrosion resistance and high purity.
Understanding the Trade-offs
Process Efficiency vs. Material Quality
The primary trade-off of furnace cooling is the extended cycle time.
Allowing a furnace to cool naturally or under controlled ramp-down rates significantly lengthens the total processing time compared to rapid cooling methods (like gas quenching). This reduces the throughput of the manufacturing process, making it more time-intensive per batch.
Equipment Demands
Maintaining a high vacuum not just during heating, but throughout a prolonged cooling phase, places stress on equipment seals and pumps.
Any leak during the cooling phase, while the material is still hot, can ruin the batch by introducing impurities. Therefore, this method requires rigorous equipment maintenance and monitoring to ensure the vacuum integrity holds until the very end.
Making the Right Choice for Your Goal
To maximize the performance of your CoCrFeNi(Cu) coatings, you must balance the cooling rate with your production requirements.
- If your primary focus is Adhesion and Structural Integrity: Prioritize a slower furnace cooling rate to minimize thermal stress and prevent delamination, particularly if the substrate and coating have vastly different thermal properties.
- If your primary focus is Corrosion Resistance and Purity: Ensure your vacuum system is capable of maintaining deep vacuum pressure (e.g., < 2 Pa) throughout the entire cooling cycle to eliminate oxidation of Chromium and Iron.
Successful protection of high-entropy alloy coatings relies on treating the cooling phase not as an afterthought, but as an active, critical stage of the manufacturing process.
Summary Table:
| Cooling Method | Key Benefit | Impact on Coating |
|---|---|---|
| Furnace Cooling | Slow, controlled temperature decrease | Prevents thermal stress & cracking |
| Vacuum Environment | No oxygen exposure | Eliminates oxidation, preserves alloy purity |
| Gradual Contraction | Matches substrate & coating CTE | Ensures strong metallurgical bond, prevents delamination |
Maximize the integrity and performance of your high-entropy alloy coatings with KINTEK's precision furnace solutions.
Our custom Muffle, Tube, Vacuum, and CVD furnaces are engineered to maintain critical vacuum integrity and precise cooling profiles, ensuring your CoCrFeNi(Cu) coatings remain crack-free and oxidation-resistant. Backed by expert R&D and manufacturing, we deliver reliable lab high-temp furnaces tailored to your unique process requirements.
Contact us today to discuss how our equipment can enhance your coating quality and yield!
Visual Guide
Related Products
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- 9MPa Air Pressure Vacuum Heat Treat and Sintering Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- 600T Vacuum Induction Hot Press Vacuum Heat Treat and Sintering Furnace
People Also Ask
- What is the purpose of setting a mid-temperature dwell stage? Eliminate Defects in Vacuum Sintering
- What is the role of a vacuum furnace in the solid-phase synthesis of TiC/Cu? Master High-Purity Material Engineering
- What is the function of a vacuum sintering furnace in the SAGBD process? Optimize Magnetic Coercivity and Performance
- Why is a vacuum environment essential for sintering Titanium? Ensure High Purity and Eliminate Brittleness
- Why is a high-vacuum environment necessary for sintering Cu/Ti3SiC2/C/MWCNTs composites? Achieve Material Purity



















