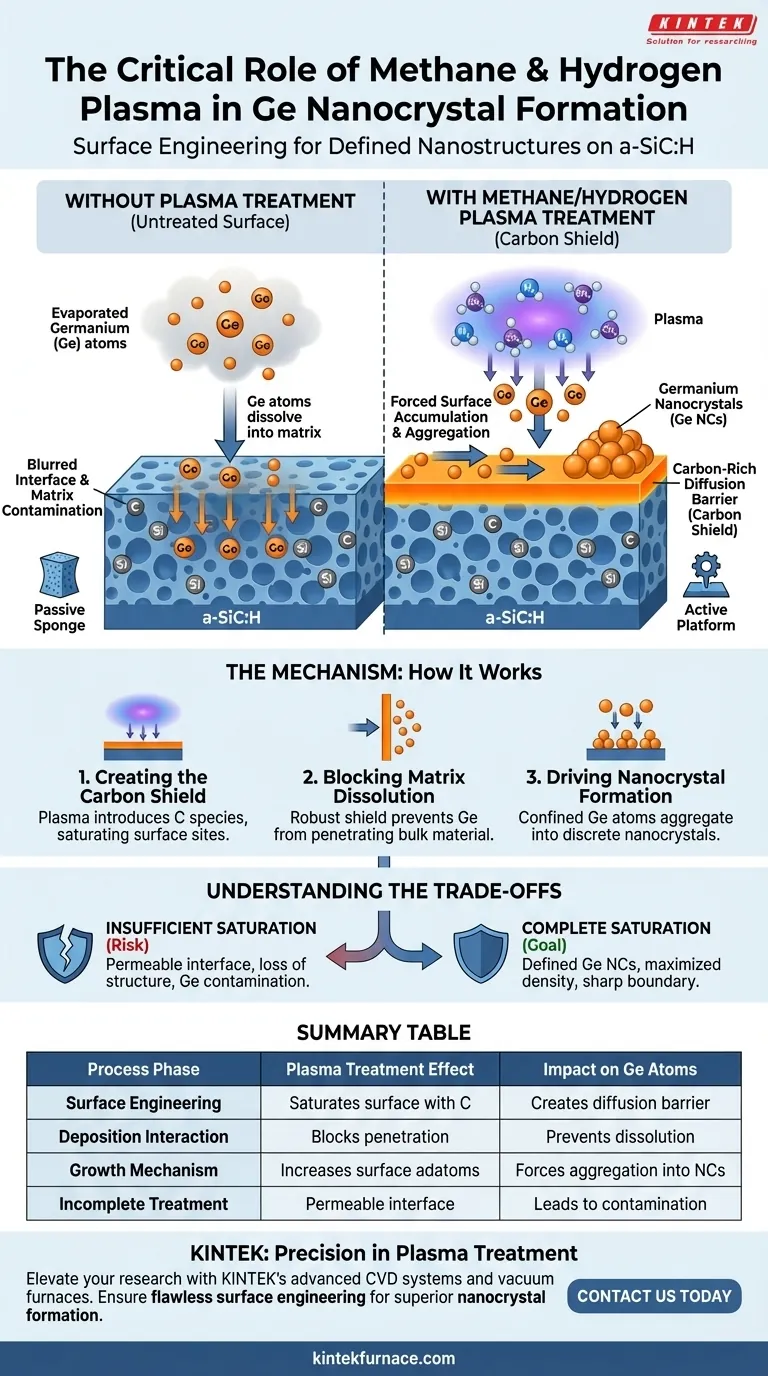
Methane and hydrogen plasma treatment acts as a critical surface engineering step to enable nanostructure growth. By exposing the hydrogenated amorphous silicon carbide (a-SiC:H) surface to this specific plasma environment, you saturate the surface with carbon atoms. This process effectively creates a chemical shield that prevents subsequently deposited germanium from dissolving into the underlying matrix, forcing it to organize into discrete nanocrystals instead.
The plasma treatment creates a carbon-rich diffusion barrier on the a-SiC:H surface. This barrier physically blocks germanium atoms from penetrating the underlying matrix, compelling them to accumulate on the surface and aggregate into stable nanocrystal structures.

The Mechanism of Surface Saturation
Creating the Carbon Shield
The primary function of the methane and hydrogen plasma is to alter the surface chemistry of the a-SiC:H. The plasma introduces carbon species that bond with and saturate the surface sites of the substrate. This results in a distinct, carbon-rich layer sitting atop the bulk material.
Blocking Matrix Dissolution
Without this specific treatment, the interface between germanium and a-SiC:H is permeable. Evaporated germanium atoms naturally tend to diffuse downward and dissolve into the amorphous silicon carbide matrix. The carbon-saturated layer acts as a robust diffusion barrier, effectively closing off this migration pathway.
Driving Nanocrystal Formation
Forcing Surface Accumulation
Because the germanium atoms are blocked from entering the bulk material, they are confined to the surface. This confinement drastically increases the concentration of germanium adatoms on top of the carbon barrier.
Promoting Aggregation
With nowhere to go but the surface, the germanium atoms are thermodynamically driven to bond with one another. This forced interaction promotes the aggregation of atoms. Consequently, rather than forming a flat alloy or dissolving, the material self-assembles into distinct, discrete germanium nanocrystals (Ge NCs).
Understanding the Trade-offs
The Risk of Insufficient Saturation
The success of this process relies entirely on the integrity of the diffusion barrier. If the plasma treatment is too brief or the carbon saturation is incomplete, the barrier will fail.
Loss of Structural Definition
In the absence of a complete barrier, germanium atoms will revert to their natural tendency to diffuse into the matrix. This leads to a loss of distinct nanocrystal features and results in germanium contamination within the a-SiC:H layer rather than the desired surface structures.
Making the Right Choice for Your Goal
To maximize the effectiveness of your germanium deposition, apply the following principles:
- If your primary focus is maximizing nanocrystal density: Ensure the plasma treatment is sufficient to fully saturate the surface, as any gaps in carbon coverage will lead to material loss into the bulk.
- If your primary focus is interface definition: Utilize the methane/hydrogen plasma to create a sharp boundary between the substrate and the active germanium layer.
By utilizing this plasma treatment, you effectively convert the substrate from a passive sponge into an active platform that supports the self-assembly of defined nanostructures.
Summary Table:
| Process Phase | Plasma Treatment Effect | Impact on Ge Atoms |
|---|---|---|
| Surface Engineering | Saturates a-SiC:H surface with carbon atoms | Creates a robust chemical diffusion barrier |
| Deposition Interaction | Blocks penetration into the underlying matrix | Prevents dissolution and material loss into bulk |
| Growth Mechanism | Increases surface adatom concentration | Forces aggregation into discrete nanocrystals |
| Incomplete Treatment | Results in a permeable or weak interface | Leads to matrix contamination and loss of structure |
Elevate Your Nanostructure Engineering with KINTEK
Precision in plasma treatment is the key to unlocking superior germanium nanocrystal formation. At KINTEK, we understand that high-performance materials require exact environmental controls. Backed by expert R&D and world-class manufacturing, we provide advanced CVD systems, vacuum furnaces, and customizable high-temperature lab solutions designed to meet your most rigorous research needs.
Whether you are refining interface definition or maximizing nanocrystal density, KINTEK offers the specialized equipment to ensure your surface engineering is flawless. Contact us today to find the perfect system for your lab!
Visual Guide
References
- Z. Remeš, Oleg Babčenko. Thin Hydrogenated Amorphous Silicon Carbide Layers with Embedded Ge Nanocrystals. DOI: 10.3390/nano15030176
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 915MHz MPCVD Diamond Machine Microwave Plasma Chemical Vapor Deposition System Reactor
- Magnesium Extraction and Purification Condensing Tube Furnace
- Multi Heating Zones CVD Tube Furnace Machine for Chemical Vapor Deposition Equipment
- Custom Made Versatile CVD Tube Furnace Chemical Vapor Deposition CVD Equipment Machine
- Inclined Rotary Plasma Enhanced Chemical Deposition PECVD Tube Furnace Machine
People Also Ask
- What is enhanced chemical vapor deposition? Achieve Low-Temperature, High-Quality Thin Films
- How does the microarc produced by ion discharge function? Enhance Coating Bonding Strength via Surface Activation
- What is Plasma-Enhanced CVD (PECVD)? Unlock Low-Temp Thin Film Deposition
- What is the role of shower head to susceptor spacing in PECVD? Optimize Film Uniformity and Deposition Rate
- What are the overall advantages of PECVD summarized? Unlock Low-Temperature, High-Quality Thin Films
- What gases are used in the PECVD system? Optimize Thin Film Deposition with Precise Gas Selection
- How does PECVD compare to thermally driven CVD processes like APCVD and LPCVD? Unlock Low-Temperature Film Deposition
- How does the method of operation in PECVD work? Unlock Low-Temperature Thin Film Deposition






