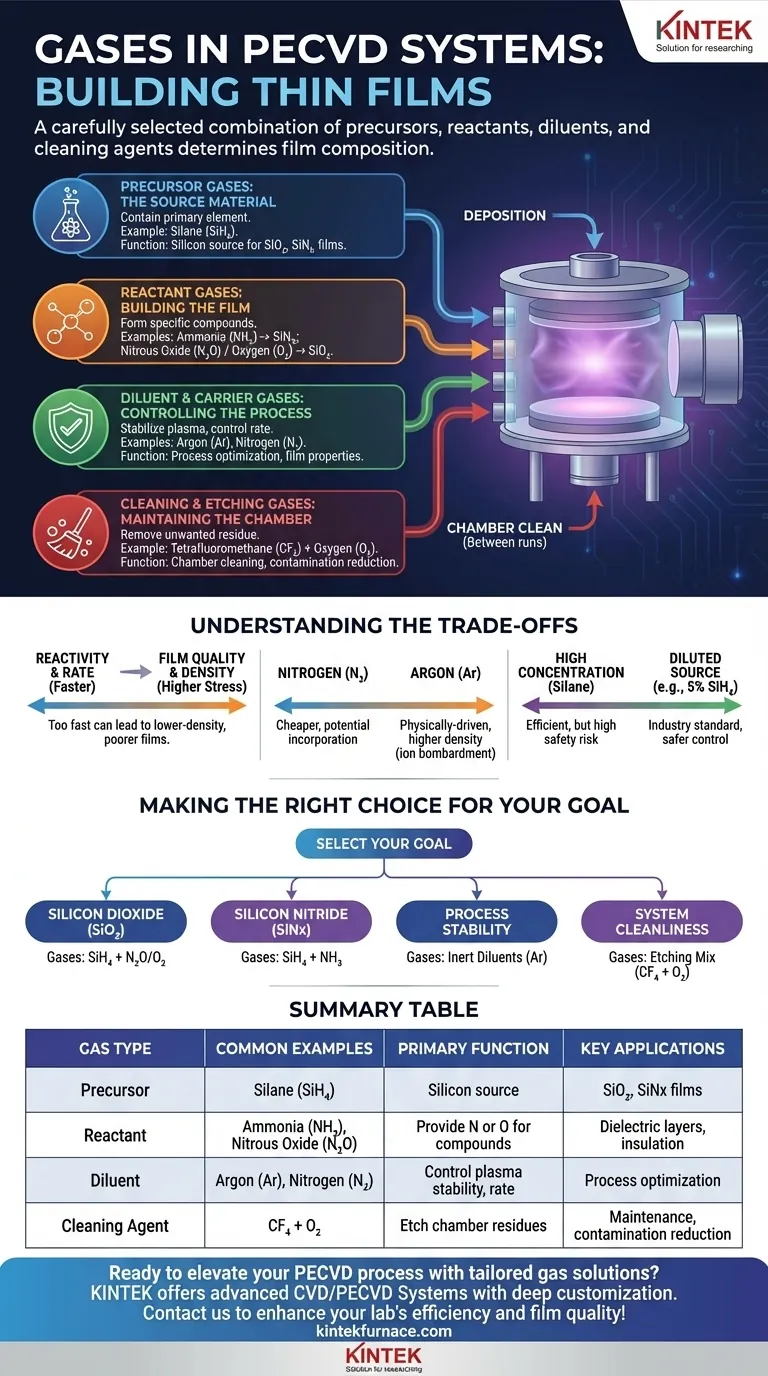
In a PECVD system, the gases used are a carefully selected combination of precursors, reactants, and diluents, chosen to build a specific thin film. Common gases include silane (SiH₄) for the silicon source, ammonia (NH₃) and nitrous oxide (N₂O) as reactants for nitrogen and oxygen, and inert gases like argon (Ar) and nitrogen (N₂) for process control. Additionally, a mixture of tetrafluoromethane (CF₄) and oxygen (O₂) is used for cleaning the chamber between depositions.
The choice of gas in Plasma-Enhanced Chemical Vapor Deposition (PECVD) is not arbitrary; it directly dictates the chemical composition of the final thin film. Each gas serves a distinct purpose as a precursor (the source material), a reactant (to form a compound), a diluent (for process control), or a cleaning agent.

The Role of Each Gas in PECVD
To understand the system, you must first understand the function of each gas. They are the fundamental building blocks of your deposition process.
Precursor Gases: The Source Material
Precursor gases contain the primary element you wish to deposit onto your substrate.
Silane (SiH₄) is the most common precursor for depositing silicon-based films, such as silicon dioxide or silicon nitride. It is highly reactive and often pyrophoric, which is why it is typically supplied in a diluted form, such as 5% SiH₄ in Nitrogen (N₂) or Argon (Ar), for safety and better process control.
Reactant Gases: Building the Film
Reactant gases are introduced with the precursor to create a specific compound material.
Ammonia (NH₃) is the standard source of nitrogen (N) atoms. It reacts with silane in the plasma to form silicon nitride (SiNx) films, which are valued for their use as dielectric layers and passivation coatings.
Nitrous Oxide (N₂O) or Oxygen (O₂) serve as the source of oxygen (O) atoms. When combined with silane, they react to form silicon dioxide (SiO₂), a critical material in microelectronics for insulation.
Diluent & Carrier Gases: Controlling the Process
These gases do not typically become part of the final film but are crucial for managing the deposition environment.
Nitrogen (N₂) and Argon (Ar) are used to dilute the reactive gases. This helps stabilize the plasma, control the deposition rate, and influence the physical properties of the film. Argon, being completely inert, does not chemically participate, while nitrogen can sometimes be incorporated into the film.
Etching & Cleaning Gases: Maintaining the Chamber
Process consistency depends on a clean chamber. Etching gases are used to remove unwanted film buildup from the chamber walls after a deposition run.
A mixture of Tetrafluoromethane (CF₄) and Oxygen (O₂), often in a 4:1 ratio, is used to generate a plasma that effectively etches away residual silicon compounds. This cleaning step is critical for ensuring process repeatability and minimizing particle contamination in subsequent runs.
Understanding the Trade-offs
The selection and ratio of gases involve critical trade-offs that directly impact the outcome of your deposition. Understanding these is key to process optimization.
Reactivity vs. Film Quality
Increasing the flow of precursor and reactant gases can increase the deposition rate, which is good for throughput. However, depositing too quickly can lead to lower-density films with poor electrical properties and higher stress.
Diluent Choice: N₂ vs. Ar
Using Argon (Ar) as a diluent gas provides a more physically-driven process, as Ar ions can bombard the film and increase its density. Using Nitrogen (N₂) is often cheaper but can be incorporated into the film unintentionally, altering its stoichiometry and properties.
Precursor Concentration vs. Safety
While a higher concentration of silane might seem efficient, it significantly increases safety risks and can make the process harder to control. Using a diluted source like 5% SiH₄ is the industry standard for balancing performance with operational safety.
Making the Right Choice for Your Goal
Your gas selection should be driven by the specific film you intend to create. Modern PECVD systems feature multiple gas lines managed by precise Mass Flow Controllers (MFCs) to allow for this flexibility.
- If your primary focus is depositing Silicon Dioxide (SiO₂): Your core gases will be a silicon precursor like SiH₄ and an oxygen source like N₂O.
- If your primary focus is depositing Silicon Nitride (SiNx): You will use a silicon precursor like SiH₄ combined with a nitrogen source like NH₃.
- If your primary focus is process stability and control: You will rely on inert diluent gases like Argon (Ar) to manage plasma density and reaction rates.
- If your primary focus is system maintenance and repeatability: You must implement a regular chamber clean using an etching gas mixture like CF₄ and O₂.
Mastering your PECVD process begins with a fundamental understanding of how each gas contributes to the final result.
Summary Table:
| Gas Type | Common Examples | Primary Function | Key Applications |
|---|---|---|---|
| Precursor | Silane (SiH₄) | Source of silicon for film deposition | Silicon dioxide, silicon nitride films |
| Reactant | Ammonia (NH₃), Nitrous Oxide (N₂O) | Provide nitrogen or oxygen to form compounds | Dielectric layers, insulation coatings |
| Diluent | Argon (Ar), Nitrogen (N₂) | Control plasma stability and deposition rate | Process optimization, film property control |
| Cleaning Agent | Tetrafluoromethane (CF₄) and Oxygen (O₂) | Etch chamber residues for cleanliness | Maintenance, contamination reduction |
Ready to elevate your PECVD process with tailored gas solutions? At KINTEK, we leverage exceptional R&D and in-house manufacturing to provide advanced high-temperature furnace solutions, including CVD/PECVD Systems. Our deep customization capabilities ensure precise alignment with your unique experimental needs, whether you're depositing silicon dioxide, silicon nitride, or optimizing process control. Don't settle for standard setups—contact us today to discuss how we can enhance your lab's efficiency and film quality!
Visual Guide
Related Products
- RF PECVD System Radio Frequency Plasma Enhanced Chemical Vapor Deposition
- Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
- Inclined Rotary Plasma Enhanced Chemical Deposition PECVD Tube Furnace Machine
- Custom Made Versatile CVD Tube Furnace Chemical Vapor Deposition CVD Equipment Machine
- Multi Heating Zones CVD Tube Furnace Machine for Chemical Vapor Deposition Equipment
People Also Ask
- What are the main components of a PECVD system? Unlock Low-Temperature Thin Film Deposition
- What is PECVD and how does it differ from traditional CVD? Unlock Low-Temperature Thin Film Deposition
- How does plasma vapor deposition work? A Low-Temperature Solution for Advanced Coatings
- What is plasma enhanced chemical vapor deposition application? Enable High-Performance Thin Films at Lower Temperatures
- How is silicon dioxide (SiO2) used in PECVD applications? Key Roles in Microfabrication



















