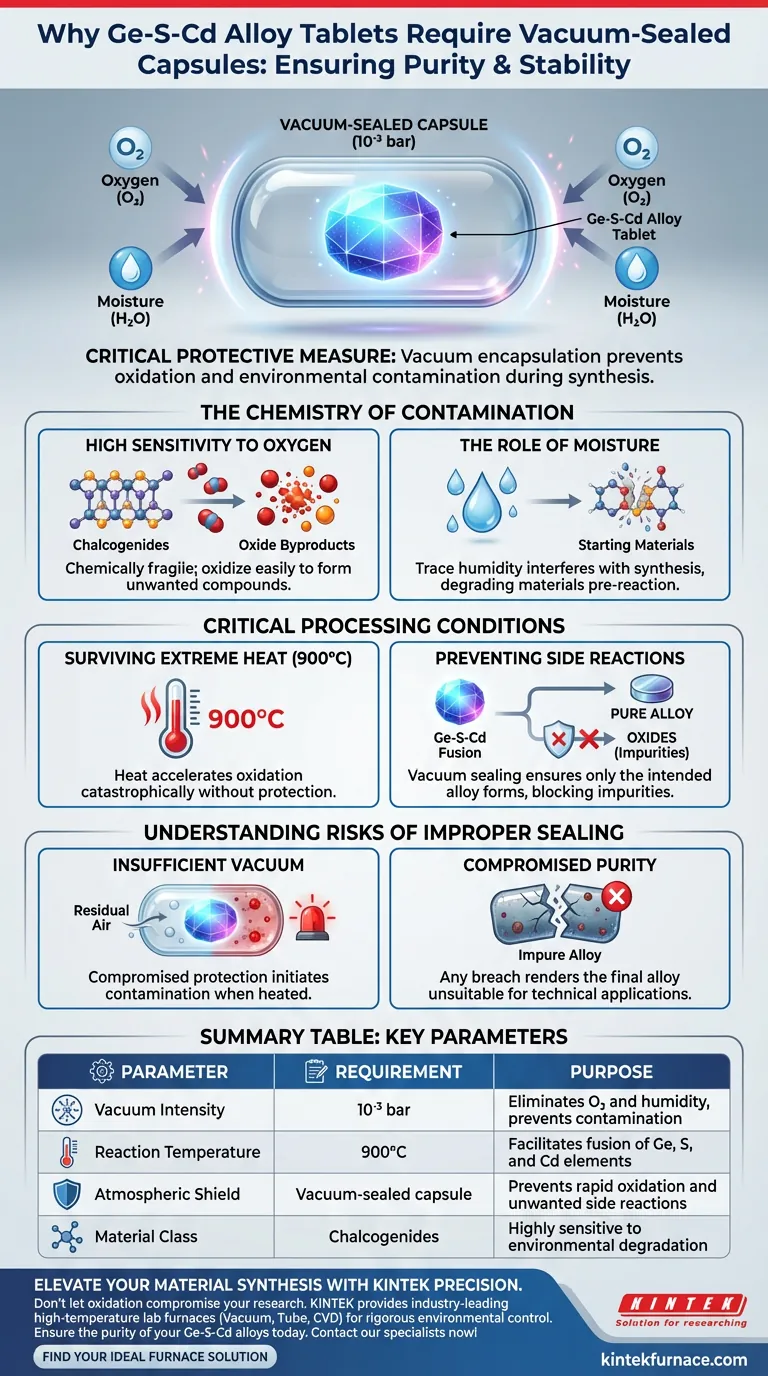
Vacuum encapsulation is a critical protective measure for maintaining the chemical integrity of Ge-S-Cd alloys during synthesis. Because chalcogenide materials are highly susceptible to oxidation, placing the pressed tablets in capsules sealed at a vacuum intensity of $10^{-3}$ bar eliminates exposure to oxygen and moisture. This isolation is mandatory to prevent environmental contamination while the material undergoes reactions at extremely high temperatures.
By removing air and humidity, vacuum sealing ensures the alloy remains pure during the 900°C reaction phase. Without this barrier, the reactive components would oxidize rapidly, compromising the material's properties and leading to unwanted chemical byproducts.

The Chemistry of Contamination
High Sensitivity to Oxygen
Chalcogenides—the chemical family to which these alloys belong—are chemically fragile when exposed to air.
They oxidize easily, meaning they react with oxygen to form new, unwanted compounds rather than the intended alloy.
The Role of Moisture
Water vapor presents a similar threat to the stability of Ge-S-Cd tablets.
Even trace amounts of humidity can interfere with the synthesis process, degrading the quality of the starting materials before the reaction even begins.
Critical Processing Conditions
Surviving Extreme Heat
The synthesis of this alloy requires a reaction temperature of 900 degrees Celsius.
Heat accelerates chemical reactions significantly. At this intensity, the rate of oxidation would be catastrophic if the material were exposed to the atmosphere.
Preventing Side Reactions
The goal of the preparation is to fuse Ge, S, and Cd into a specific alloy structure.
Vacuum sealing ensures that this is the only reaction that occurs. It blocks "side reactions," such as the formation of oxides, which would act as impurities in the final product.
Understanding the Risks of Improper Sealing
The Cost of Insufficient Vacuum
If the vacuum intensity does not meet the $10^{-3}$ bar standard, the protection is compromised.
Residual air left inside a poorly evacuated capsule provides enough oxygen to initiate contamination once the temperature rises.
Compromised Purity
The success of this preparation method hinges entirely on purity.
Any breach in the vacuum seal allows environmental contaminants to enter, rendering the final alloy impure and likely unsuitable for its intended technical application.
Ensuring Successful Alloy Preparation
To achieve high-purity Ge-S-Cd alloys, adherence to strict environmental controls is essential during the encapsulation phase.
- If your primary focus is material purity: Ensure the vacuum intensity reaches at least $10^{-3}$ bar to guarantee an environment absolutely free of oxygen and moisture.
- If your primary focus is process stability: Verify capsule integrity prior to heating to 900°C to prevent catastrophic oxidation during the critical reaction phase.
Rigorous vacuum encapsulation is the only way to safeguard chalcogenides against their natural tendency to oxidize under the intense heat required for alloy formation.
Summary Table:
| Parameter | Requirement | Purpose |
|---|---|---|
| Vacuum Intensity | 10⁻³ bar | Eliminates oxygen and humidity to prevent contamination |
| Reaction Temperature | 900°C | Facilitates fusion of Ge, S, and Cd elements |
| Atmospheric Shield | Vacuum-sealed capsule | Prevents rapid oxidation and unwanted side reactions |
| Material Class | Chalcogenides | Highly sensitive to environmental degradation |
Elevate Your Material Synthesis with KINTEK Precision
Don't let oxidation compromise your research. KINTEK provides industry-leading high-temperature lab furnaces—including Vacuum, Tube, and CVD systems—specifically designed to maintain the rigorous environmental controls required for chalcogenide and alloy preparation. Backed by expert R&D and manufacturing, our systems are fully customizable to meet your unique synthesis needs.
Ensure the purity of your Ge-S-Cd alloys today. Contact our specialists now to find your ideal furnace solution!
Visual Guide
References
- Zainab Abd Al-hadi, Kareem A. Jasim. The Effect of Partial Substitution of Ge-S-Cd Alloys on the Density of Energy States. DOI: 10.30526/37.1.3314
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Split Chamber CVD Tube Furnace with Vacuum Station CVD Machine
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Hot Press Furnace Machine for Lamination and Heating
- Vacuum Heat Treat Sintering and Brazing Furnace
- Molybdenum Vacuum Heat Treat Furnace
People Also Ask
- How does vacuum heat treatment enhance product quality? Achieve Superior Material Integrity and Performance
- How does vacuum annealing and tempering improve material properties? Enhance Strength, Purity, and Durability
- How does a vacuum distillation system function in REE extraction? Master LME Separation with Precision
- Why is a vacuum drying oven necessary for M-S-H hydration analysis? Ensure Structural Integrity & Data Accuracy
- How is a vacuum furnace energy-efficient? Uncover Key Mechanisms for Lower Costs
- What are the technical advantages of using a vacuum drying oven? Protect WC-Co-Ni Powders from Oxidation
- Why is a high-vacuum heat treatment furnace necessary for vacuum annealing HEA coatings? Ensure Chemical Stability
- What are the key characteristics of vacuum hardening? Achieve Clean, Precise Metal Hardening for Superior Components












