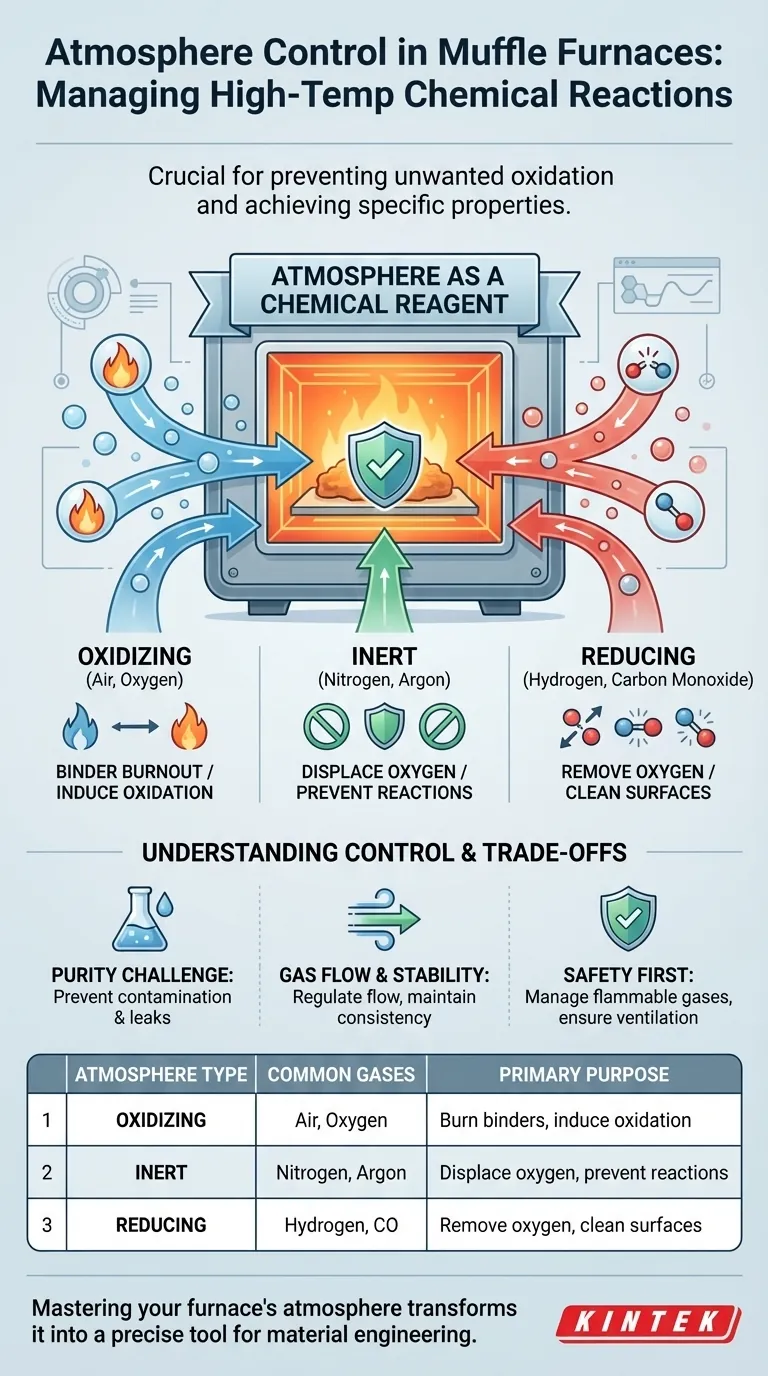
At its core, atmosphere control in a muffle furnace is about managing chemical reactions at high temperatures. It is essential because the gas surrounding your sample is not inert; it actively participates in the process, and controlling it is crucial for preventing unwanted outcomes like oxidation and for achieving specific, desirable material properties. The primary atmospheres used are oxidizing (air), inert (nitrogen, argon), and reducing (hydrogen, carbon monoxide).
The atmosphere inside a furnace should not be viewed as empty space, but as a critical chemical reagent. Failing to control it is like allowing an unknown ingredient into your experiment—it makes the final result unpredictable and unrepeatable.

The Role of the Atmosphere as a Chemical Reagent
Many users focus solely on temperature and time, overlooking the powerful chemical influence of the furnace environment. At elevated temperatures, the gas atmosphere becomes highly reactive and directly impacts your material's surface and internal structure.
Beyond Just Heat: Why the Gas Matters
Heating a material energizes its atoms, making them more susceptible to chemical change. The gases present in the furnace—whether intentionally introduced or simply ambient air—will react with the heated material. This control over chemical reactions is the fundamental reason atmosphere control is so important for quality and consistency.
Preventing Unwanted Oxidation
For most metals and many advanced materials, heating in the presence of oxygen (air) is destructive. This process, called oxidation, creates a brittle, discolored scale on the surface, altering the material's dimensions, finish, and mechanical properties. A controlled atmosphere is used to displace this oxygen and protect the workpiece.
Inducing Desired Chemical Changes
Conversely, sometimes a specific chemical reaction is the goal. A reducing atmosphere, for example, is used to strip oxygen atoms from a material's surface. This can clean away existing oxides or alter the chemical composition of a compound in a process known as chemical reduction.
A Breakdown of Common Furnace Atmospheres
The choice of atmosphere depends entirely on your process goal. Each type serves a distinct chemical purpose.
Oxidizing Atmosphere (Air, Oxygen)
This is the default environment in any furnace not sealed and purged with another gas. An oxidizing atmosphere is rich in oxygen.
While often undesirable for metals, it is intentionally used in processes like binder burnout in ceramics and powder metallurgy, where an organic binding agent must be burned away cleanly before the final sintering step.
Inert Atmosphere (Nitrogen, Argon)
An inert atmosphere provides a neutral environment. Gases like nitrogen and argon are non-reactive with most materials, even at very high temperatures.
Their sole purpose is to displace oxygen and prevent any chemical reactions from occurring, whether oxidizing or reducing. This is ideal for processes like annealing or sintering where you need to preserve the material's surface chemistry and finish.
Reducing Atmosphere (Hydrogen, Carbon Monoxide)
A reducing atmosphere actively works to remove oxygen. Gases like hydrogen are powerful reducing agents that readily bond with oxygen atoms, pulling them off the surface of the workpiece.
This is critical for heat-treating oxygen-sensitive alloys, brazing without flux, or any process where even trace amounts of surface oxide can compromise the final product's quality and performance.
Understanding the Trade-offs and Control
Implementing atmosphere control introduces complexity that must be managed carefully for successful and safe operation.
The Challenge of Purity
Achieving a truly pure inert or reducing atmosphere is a significant challenge. Any leaks in the furnace seals or gas lines can allow ambient air to contaminate the environment, negating the benefits of the controlled gas. This is why specialized atmosphere furnaces are designed with superior seals.
Gas Flow and Stability
Control is not just about filling the furnace with a gas; it's about maintaining a stable and consistent environment. A gas flow meter is used to regulate a continuous, low-pressure flow of gas through the furnace. This steady flow purges any contaminants and ensures the atmosphere remains consistent throughout the heating cycle.
Safety with Flammable Gases
Reducing gases like hydrogen and carbon monoxide are flammable and pose a safety risk. Systems using these gases require robust safety protocols, including proper ventilation, leak detection, and carefully managed purging cycles to ensure explosive mixtures with air are not created inside the furnace.
Choosing the Right Atmosphere for Your Process
Your specific goal dictates the appropriate atmospheric environment. There is no single "best" atmosphere; there is only the right one for the job.
- If your primary focus is firing basic ceramics or burning out binders: An oxidizing atmosphere (air) is often sufficient and the simplest to implement.
- If your primary focus is annealing or sintering metals without surface scaling: An inert atmosphere (nitrogen or argon) is necessary to protect the material from oxygen.
- If your primary focus is cleaning surface oxides or performing advanced heat treatment: A reducing atmosphere (a non-flammable hydrogen/nitrogen blend) is required to actively deoxidize the material.
Mastering your furnace's atmosphere transforms it from a simple oven into a precise tool for material engineering.
Summary Table:
| Atmosphere Type | Common Gases | Primary Purpose |
|---|---|---|
| Oxidizing | Air, Oxygen | Burn binders, induce oxidation |
| Inert | Nitrogen, Argon | Displace oxygen, prevent reactions |
| Reducing | Hydrogen, Carbon Monoxide | Remove oxygen, clean surfaces |
Unlock the full potential of your lab with KINTEK's advanced high-temperature furnace solutions! Leveraging exceptional R&D and in-house manufacturing, we provide diverse laboratories with reliable muffle, tube, rotary, vacuum, atmosphere furnaces, and CVD/PECVD systems. Our strong deep customization capability ensures we precisely meet your unique experimental needs, enhancing efficiency and results. Contact us today to discuss how we can support your specific requirements!
Visual Guide
Related Products
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- What is the use of nitrogen in furnace? Prevent Oxidation for Superior Heat Treatment
- How does the inert atmosphere heat treating process work? Prevent Oxidation for Superior Material Quality
- What are the benefits of inert atmosphere heat treating? Prevent Oxidation and Preserve Material Integrity
- What is nitrogen used for in a furnace? Prevent Oxidation and Control Heat Treatment Quality
- What are the environmental benefits of using inert gases in furnaces? Reduce Waste and Emissions for a Greener Process



















