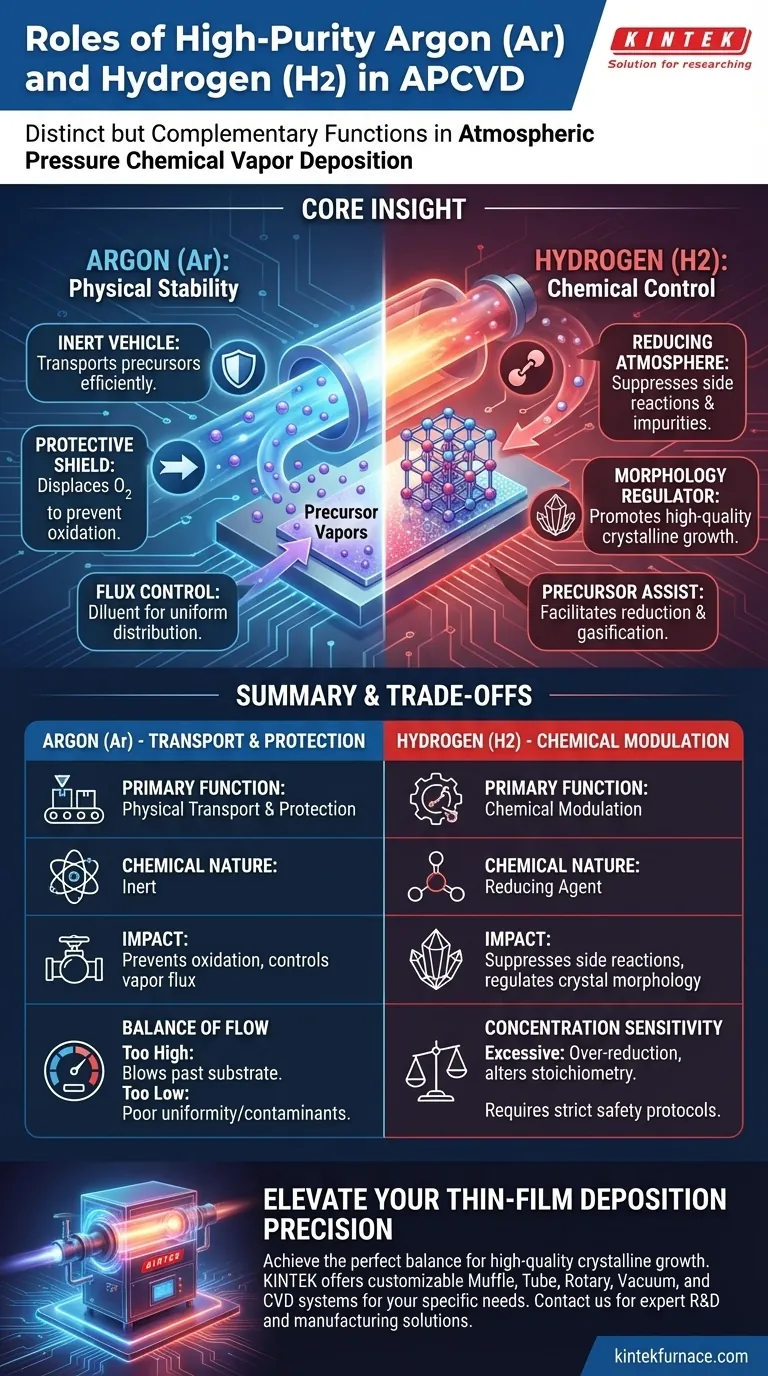
High-purity Argon (Ar) and Hydrogen (H2) serve distinct but complementary functions in Atmospheric Pressure Chemical Vapor Deposition (APCVD). Argon acts as the primary physical transport medium, creating an inert environment that moves precursor vapors to the substrate while preventing oxidation. Hydrogen functions as an active chemical agent, providing a reducing atmosphere that suppresses side reactions and regulates the morphological evolution of the final crystal structure.
Core Insight: While Argon provides the necessary physical stability for material transport, Hydrogen provides the chemical control required to refine crystal quality. The precise ratio and flow of these gases determine the purity and structural integrity of the deposited film.

The Role of Argon (Ar): Transport and Protection
The Inert Physical Vehicle
Argon functions as the "vehicle" in the deposition process. It is responsible for the transport of sublimated precursor vapors from the source zone to the downstream substrate.
By utilizing precise flow control, Argon ensures the reactants reach the deposition zone efficiently. This flow determines the concentration gradient of reactants available at the substrate surface.
Preventing Oxidation
The primary chemical role of Argon is its inertness. It creates a protective atmosphere by displacing air and oxygen from the furnace tube.
This is critical for preventing the unintended oxidation of both the precursor materials and the growing film. Without this inert shield, high temperatures would degrade the materials before deposition could occur.
Controlling Vapor Flux
Argon also acts as a diluent. By adjusting the flow rate, you can precisely control the vapor flux—the amount of material reaching the substrate per unit of time.
This regulation prevents precursor backflow and ensures a uniform distribution of vapors, directly influencing the film's growth rate and uniformity.
The Role of Hydrogen (H2): Chemical Modulation
Creating a Reducing Atmosphere
Unlike Argon, Hydrogen is chemically active. It is introduced to create a reducing atmosphere within the reaction chamber.
This environment helps suppress unwanted side reactions that could introduce impurities into the film. It essentially "cleans" the chemical pathway, ensuring the reaction proceeds toward the desired product.
Regulating Crystal Morphology
Hydrogen plays a critical role in determining the physical shape and quality of the final product. It regulates the morphological evolution of the crystals (such as SnSe2 or SnSe).
By modifying the surface energy and reaction kinetics, Hydrogen promotes high-quality crystalline growth. It helps define the texture and structure of the deposited material, preventing amorphous or disordered growth.
Assisting Precursor Reduction
In specific processes involving oxide precursors (like In2O3), Hydrogen assists in reduction and gasification.
This ensures the precursor breaks down correctly to release the necessary elements for deposition, facilitating the formation of pure-phase materials.
Understanding the Trade-offs
The Balance of Flow Rates
While Argon flow is necessary for transport, an excessive flow rate can be detrimental. High velocity can blow precursors past the substrate before they have time to react and deposit. Conversely, a flow that is too low may result in poor uniformity or back-diffusion of contaminants.
Hydrogen Concentration Sensitivity
Hydrogen is powerful but must be used sparingly. Typically, it is introduced as a mixture (e.g., 5% H2 in Ar).
Excessive Hydrogen can lead to over-reduction of the substrate or precursor, altering the stoichiometry of the final film. Furthermore, managing Hydrogen at high temperatures introduces safety complexities that require rigorous handling protocols compared to pure inert gases.
Making the Right Choice for Your Goal
To optimize your APCVD process, align your gas strategy with your specific defects:
- If your primary focus is Phase Purity: Prioritize the stability of the Argon flow to ensure total exclusion of oxygen and consistent precursor transport.
- If your primary focus is Crystal Quality: Fine-tune the Hydrogen concentration to strictly regulate the reaction atmosphere and improve surface morphology.
- If your primary focus is Film Uniformity: Adjust the Argon carrier flow rate to modify the vapor flux and concentration gradient across the substrate.
Success in APCVD relies on using Argon to stabilize the environment and Hydrogen to refine the chemistry.
Summary Table:
| Gas Type | Primary Function | Chemical Nature | Impact on Process |
|---|---|---|---|
| Argon (Ar) | Physical Transport & Protection | Inert | Prevents oxidation and controls vapor flux/dilution. |
| Hydrogen (H2) | Chemical Modulation | Reducing Agent | Suppresses side reactions and regulates crystal morphology. |
Elevate Your Thin-Film Deposition Precision
Achieving the perfect balance between Argon transport and Hydrogen modulation is the key to high-quality crystalline growth. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems—all fully customizable to meet your specific lab research and industrial production needs.
Whether you are refining 2D materials or optimizing semiconductor films, our technical team is ready to help you configure the ideal furnace for your APCVD process.
Contact KINTEK Today to Customize Your High-Temp Solution
Visual Guide
References
- Manab Mandal, K. Sethupathi. In Situ Simultaneous Growth of Layered SnSe<sub>2</sub> and SnSe: a Linear Precursor Approach. DOI: 10.1002/admi.202500239
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Magnesium Extraction and Purification Condensing Tube Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- Vertical Laboratory Quartz Tube Furnace Tubular Furnace
People Also Ask
- What is inside-out processing in CVD? Build Complex, Hollow Components with Precision
- What future trends are expected in the development of CVD tube furnaces? Discover Smarter, More Versatile Systems
- What are the technical advantages of using a CVD system? Optimize Carbon Nanotube Growth for Thermal Conductivity
- Why is high-precision gas flow control essential for the CVD of graphene-palladium? Master Material Quality Control
- What are the advantages of using in-situ CVD for Ag-ZIF-8/Ni foam? Enhance Structural Stability & Uniformity
- What is the operating principle of a Quartz Crystal Thickness Monitor? Achieve Precise ZTO Thin Film Control
- What are the key application fields of CVD tube furnaces? Unlock Precision in Thin-Film Synthesis
- Can CVD furnaces be combined with other technologies? If so, how? Unlock Advanced Material Engineering









