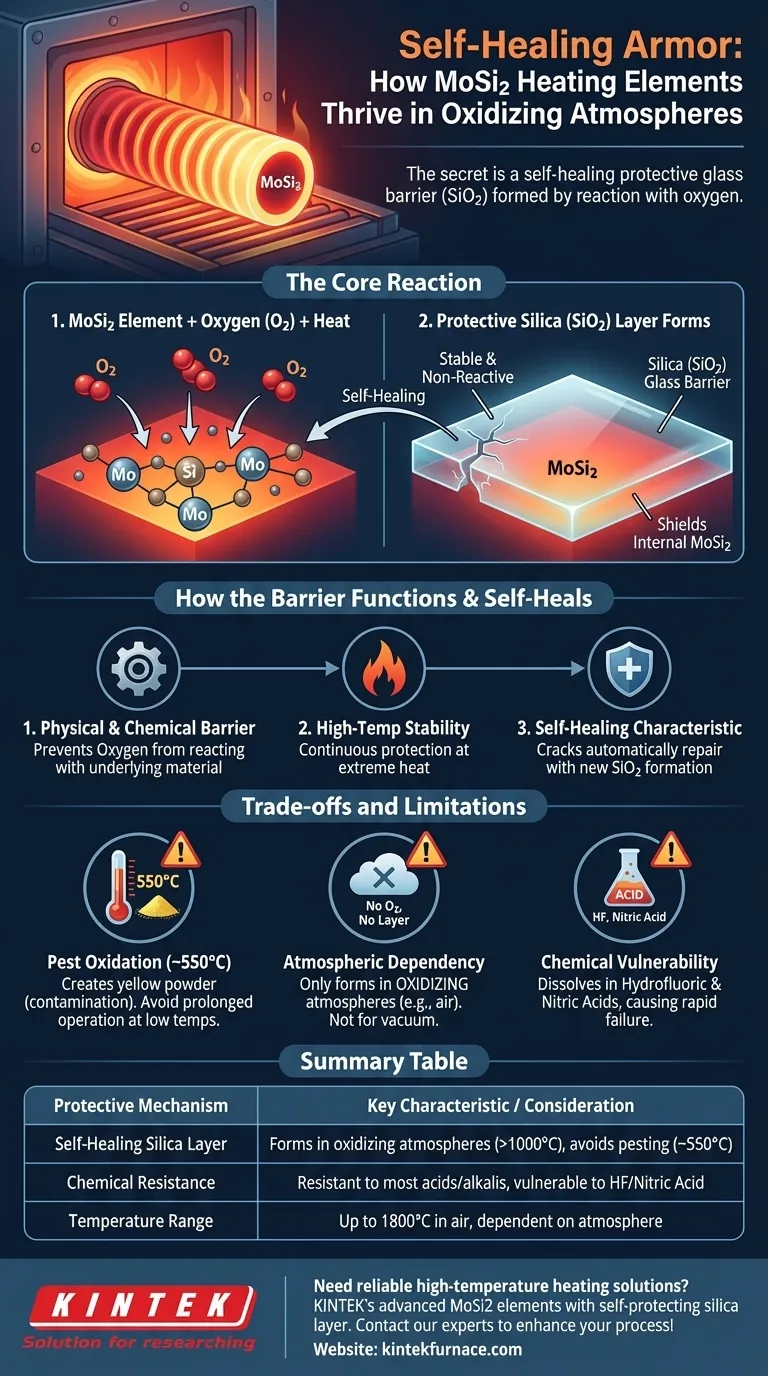
In an oxidizing atmosphere, the protection for Molybdenum Disilicide (MoSi2) heating elements comes from a self-healing process. When exposed to oxygen at high temperatures, the silicon in the MoSi2 compound reacts to form a thin, protective layer of silica (SiO2), or glass, on its surface. This passive layer is what prevents the underlying material from further oxidation and enables its long service life.
The core principle is self-preservation through controlled reaction. Instead of being destroyed by oxygen, MoSi2 uses it to create a durable, non-reactive glass barrier that shields it from further attack, effectively "healing" its own surface.

How the Protective Layer Forms and Functions
The Core Reaction
When a MoSi2 element heats up in the presence of oxygen, a chemical reaction occurs. The silicon (Si) on the surface combines with oxygen (O2) from the atmosphere.
This reaction forms a thin, continuous film of silica (SiO2). This silica layer is essentially a form of glass that is highly stable and non-reactive.
The Role of the Silica (SiO2) Barrier
This newly formed SiO2 layer acts as a physical and chemical barrier. It prevents oxygen from reaching and reacting with the underlying MoSi2 material.
Because the layer is stable at very high temperatures, it provides continuous protection, allowing the element to operate effectively in environments where other materials would quickly degrade.
The Self-Healing Characteristic
If the protective silica layer is damaged or cracked, the self-healing process restarts. The newly exposed MoSi2 surface will immediately react with the surrounding oxygen to form new SiO2, effectively repairing the breach.
This regenerative capability is what gives MoSi2 elements their exceptional durability and long lifespan in high-temperature, oxidizing environments.
Understanding the Trade-offs and Limitations
While robust, the protective mechanism of MoSi2 is not without its limitations. Understanding these is critical for proper application and to avoid premature failure.
The "Pest Oxidation" Phenomenon
At lower temperatures, specifically around 550°C (1022°F), MoSi2 can experience a different type of oxidation known as "pest oxidation" or "pesting."
This process creates a yellowish powder on the element's surface. While this low-temperature oxidation does not typically impact the element's performance, the resulting powder can become a source of contamination for the products being heated.
Therefore, prolonged operation in this specific low-temperature range should be avoided to maintain a clean furnace environment.
Atmospheric Dependencies
The maximum operating temperature of MoSi2 elements is highly dependent on the atmosphere. The self-healing silica layer only forms in an oxidizing atmosphere, such as air.
In non-air or vacuum environments, this protective layer cannot form, which alters the material's operational limits and behavior.
Chemical Vulnerability
The silica layer, while resistant to most acids and alkalis, is not invincible. It will dissolve when exposed to hydrofluoric acid and nitric acid. Using MoSi2 elements in processes involving these chemicals will lead to rapid degradation and failure.
How to Apply This to Your Process
Understanding this mechanism helps ensure you use MoSi2 elements correctly for maximum lifespan and performance.
- If your primary focus is high-temperature stability: Ensure your process runs in an oxidizing atmosphere (like air) to allow the protective SiO2 layer to form and regenerate.
- If your primary focus is product purity: Avoid dwelling in the 550°C temperature range to prevent "pest oxidation" and the formation of contaminating powder.
- If your primary focus is chemical processing: Verify that your process atmosphere is free from hydrofluoric acid or nitric acid, which will destroy the element's protective layer.
Ultimately, the effectiveness of a MoSi2 heating element is directly tied to managing the conditions that allow its protective glass layer to thrive.
Summary Table:
| Protective Mechanism | Key Characteristic | Important Consideration |
|---|---|---|
| Self-Healing Silica Layer | Forms in oxidizing atmospheres (>1000°C) | Avoid low temperatures (~550°C) to prevent pest oxidation |
| Chemical Resistance | Resistant to most acids/alkalis | Vulnerable to HF and nitric acid |
| Temperature Range | Up to 1800°C in air | Maximum temperature depends on atmosphere |
Need reliable high-temperature heating solutions for your laboratory? KINTEK's advanced MoSi2 heating elements deliver exceptional performance in oxidizing atmospheres through their self-protecting silica layer. Leveraging our strong R&D and in-house manufacturing capabilities, we provide precisely engineered tube furnaces, vacuum furnaces, and CVD/PECVD systems with deep customization to meet your unique experimental requirements. Contact our experts today to discuss how our heating solutions can enhance your process efficiency and longevity!
Visual Guide
Related Products
- Silicon Carbide SiC Thermal Heating Elements for Electric Furnace
- Molybdenum Vacuum Heat Treat Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Laboratory Muffle Oven Furnace with Bottom Lifting
- Split Multi Heating Zone Rotary Tube Furnace Rotating Tube Furnace
People Also Ask
- Why is silicon carbide resistant to chemical reactions in industrial furnaces? Unlock Durable High-Temp Solutions
- What are the advantages of using high purity green silicon carbide powder in heating elements? Boost Efficiency and Lifespan
- What are the properties and applications of silicon carbide (SiC)? Unlock High-Temperature Performance
- Why are SiC heating elements considered environmentally friendly? Discover Their Eco-Efficiency & Lifespan Insights
- What are the properties and capabilities of Silicon Carbide (SiC) as a heating element? Unlock Extreme Heat and Durability



















