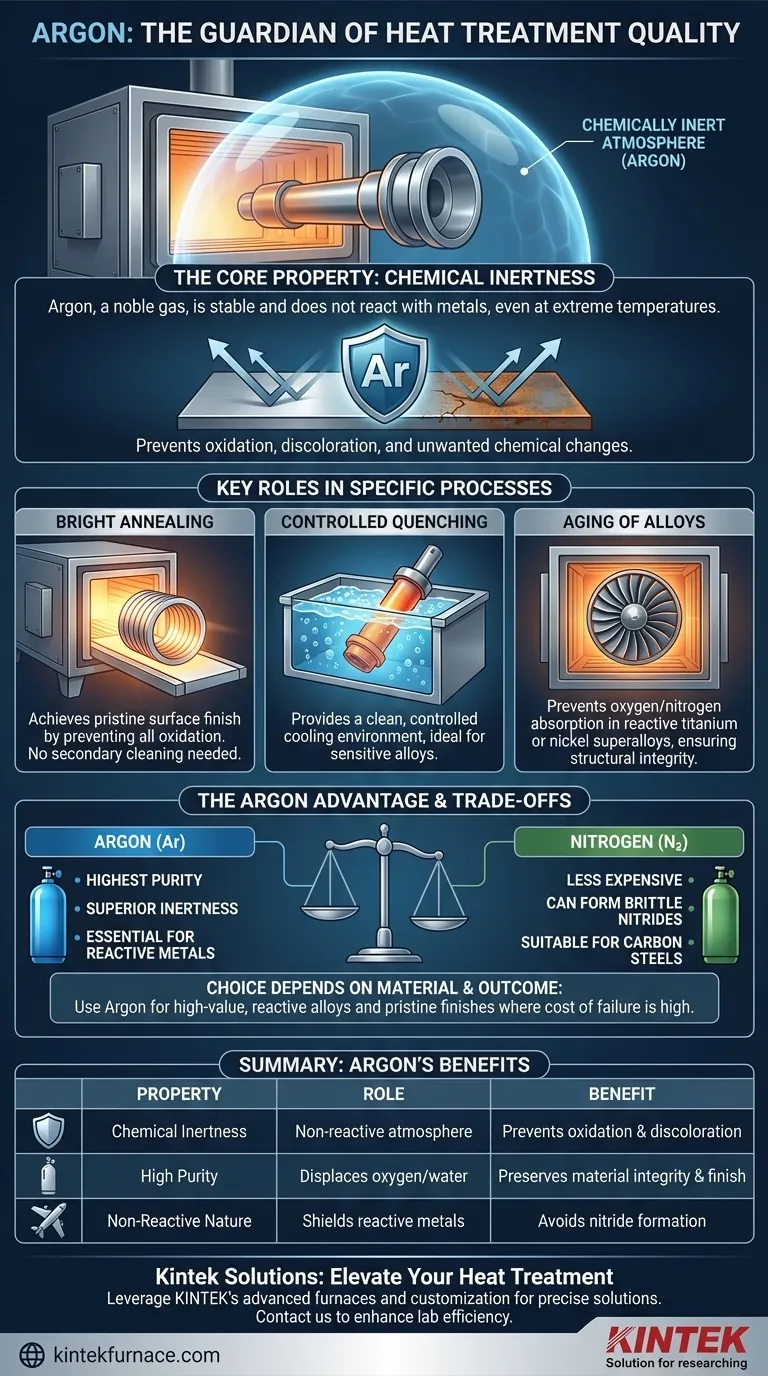
At its core, argon is ideal for heat treatment because it is a chemically inert gas. This fundamental property means it will not react with metals even at extreme temperatures, creating a protective atmosphere that prevents oxidation, discoloration, and other unwanted chemical changes that would otherwise compromise the material's quality.
The primary role of argon in heat treatment is not to act upon the metal, but to prevent anything else—specifically oxygen—from doing so. It serves as an invisible, non-reactive shield, ensuring the material's intended chemical and mechanical properties are perfectly preserved.

The Foundation: Chemical Inertness
Argon's value is rooted in its status as a noble gas. Its atomic structure makes it exceptionally stable and unwilling to form chemical bonds with other elements.
What "Inert" Means in Practice
In a heat treatment furnace, high temperatures act as a catalyst for chemical reactions. An inert atmosphere, created by flooding the chamber with argon, displaces reactive gases like oxygen and water vapor.
This effectively creates a chemically sterile environment around the metal part, preventing any reactions from occurring on its surface.
Preventing Oxidation and Discoloration
The most common unwanted reaction at high temperatures is oxidation. For steels, this results in scaling; for other metals, it causes discoloration and the formation of a brittle oxide layer.
By displacing oxygen, argon ensures the metal's surface remains clean and bright. This is critical in processes like "bright annealing," where a pristine surface finish is a primary goal.
Preserving Material Integrity
Beyond surface appearance, unwanted reactions can alter the fundamental properties of the metal. They can change the alloy's chemical composition at the surface, affecting its strength, ductility, and fatigue resistance.
Argon's inert shield guarantees that the heat treatment process modifies the metal's physical structure (its microstructure) without altering its chemical makeup.
Argon in Specific Heat Treatment Processes
The principle of inerting is applied across several critical high-temperature processes to ensure predictable, high-quality results.
Bright Annealing
In annealing, the goal is to soften a metal and relieve internal stresses. Using an argon atmosphere prevents any surface oxidation, resulting in a part that is both structurally sound and has a bright, clean finish without needing secondary cleaning operations.
Controlled Quenching
Quenching involves rapid cooling to lock in a desired material structure. Using argon as the quenching medium provides a clean, controlled cooling environment, which is especially important for sensitive alloys that could be contaminated by other cooling agents.
Aging of High-Performance Alloys
Specialty alloys, such as those based on titanium or nickel, are often heat-treated to develop specific properties through a process called aging. These materials are extremely reactive at high temperatures.
An argon atmosphere is essential to prevent the absorption of oxygen or nitrogen, which would degrade their structural integrity and performance in critical applications like aerospace components.
Understanding the Trade-offs: Argon vs. Other Atmospheres
While effective, argon is not the only option for creating a protective atmosphere. The decision often involves balancing performance requirements with cost.
The Purity of Argon
Argon provides one of the highest levels of inertness available for industrial processes. It is more inert than nitrogen, especially at very high temperatures.
When to Choose Argon Over Nitrogen
Nitrogen is a common and less expensive alternative. However, at high temperatures, nitrogen can react with certain metals like titanium, aluminum, and some high-strength stainless steels to form nitrides.
These nitrides can make the surface brittle and are often undesirable. In these cases, argon is the only safe choice to guarantee a completely non-reactive environment.
Cost vs. Performance
Argon is generally more expensive to produce and use than nitrogen. Therefore, its use is typically reserved for applications where the cost of material failure or compromised quality is high.
For general-purpose heat treatment of common carbon steels, a nitrogen-based atmosphere is often sufficient and more economical.
Making the Right Choice for Your Process
Selecting the right atmosphere depends entirely on the material being treated and the required outcome.
- If your primary focus is treating high-value, reactive alloys (like titanium or nickel-based superalloys): Argon is the necessary choice to prevent nitride formation and ensure maximum material integrity.
- If your primary focus is achieving a perfectly clean, bright surface finish on stainless steel: Argon is the superior option for bright annealing, guaranteeing no discoloration.
- If your primary focus is cost-effective heat treatment of standard carbon or low-alloy steels: A nitrogen-based atmosphere is typically sufficient and more economical.
Ultimately, using argon is an investment in certainty, ensuring the heat treatment process delivers exactly the properties you designed it to achieve.
Summary Table:
| Property | Role in Heat Treatment | Key Benefit |
|---|---|---|
| Chemical Inertness | Creates a non-reactive atmosphere | Prevents oxidation and discoloration |
| High Purity | Displaces oxygen and water vapor | Preserves material integrity and surface finish |
| Non-Reactive Nature | Shields reactive metals like titanium | Avoids nitride formation and brittleness |
Upgrade your heat treatment processes with KINTEK's advanced furnace solutions! Leveraging exceptional R&D and in-house manufacturing, we provide diverse laboratories with high-temperature furnaces like Muffle, Tube, Rotary, Vacuum & Atmosphere, and CVD/PECVD Systems. Our deep customization capabilities ensure precise solutions for your unique experimental needs, guaranteeing superior performance and material quality. Contact us today to discuss how we can enhance your lab's efficiency and results!
Visual Guide
Related Products
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
People Also Ask
- Can box type high-temperature resistance furnaces control the atmosphere? Unlock Precision in Material Processing
- How do argon and nitrogen protect samples in vacuum furnaces? Optimize Your Thermal Process with the Right Gas
- What are the development prospects of atmosphere box furnaces in the aerospace industry? Unlock Advanced Material Processing for Aerospace Innovation
- What is an atmosphere protection muffle furnace? Unlock Precise Heat Treatment in Controlled Environments
- How does a mixed gas flow control system maintain stability during high-temperature nitriding? Precision Gas Ratios



















