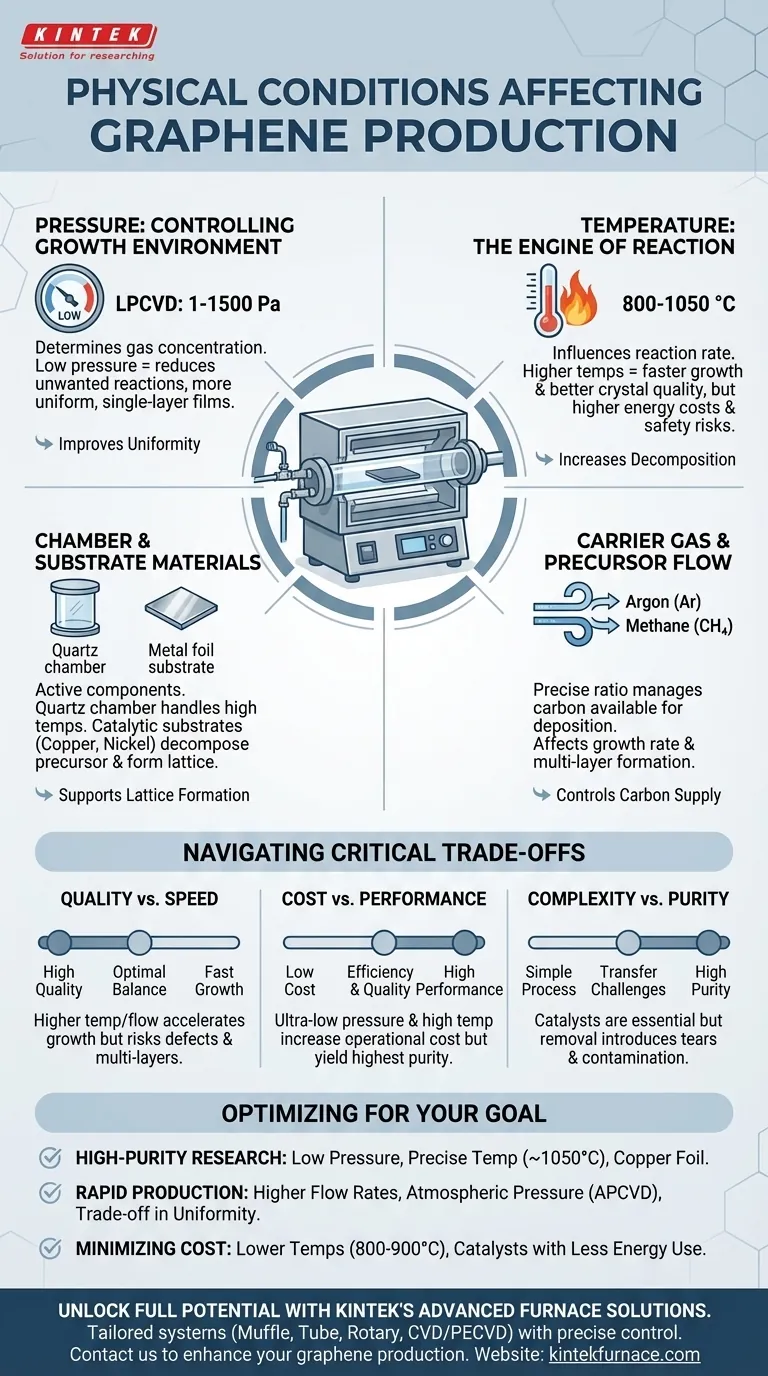
The quality and scalability of graphene production are directly governed by a precise set of physical conditions. The most critical factors are the synthesis temperature, chamber pressure, carrier gas flow, and the choice of catalytic materials. These variables must be meticulously controlled within a Chemical Vapor Deposition (CVD) system to achieve the desired material properties.
Achieving high-quality graphene is not about maximizing a single variable, but about carefully tuning the interplay between pressure, temperature, and catalysts to control the carbon deposition process. Each parameter presents a critical trade-off between growth speed, material quality, and operational cost.

The Core Variables of Graphene Synthesis
The foundation of modern graphene production, particularly via CVD, rests on manipulating a few key physical parameters. Each one acts as a lever that can be adjusted to influence the final outcome.
Temperature: The Engine of the Reaction
The synthesis temperature directly dictates the rate of the chemical reactions involved in graphene formation.
Most processes operate in a range of 800 to 1050 °C. Higher temperatures increase the decomposition rate of the carbon precursor gas (like methane) and the surface mobility of carbon atoms, which generally leads to faster growth and higher quality crystals.
However, operating at the upper end of this range significantly increases energy costs and introduces safety considerations for the equipment and lab environment.
Pressure: Controlling the Growth Environment
Chamber pressure determines the concentration of gas molecules and influences the uniformity of the deposition.
The two main approaches are Low-Pressure CVD (LPCVD) and Atmospheric Pressure CVD (APCVD). Most high-quality synthesis relies on LPCVD, with pressures typically between 1 and 1500 Pascals.
Low pressures are favored because they reduce the likelihood of unwanted gas-phase reactions. This leads to a more controlled, surface-mediated growth process, resulting in more uniform, single-layer graphene films.
Carrier Gas and Precursor Flow
A carrier gas, typically Argon (Ar) or Hydrogen (H₂), is used to transport the carbon source (precursor) into the reaction chamber and over the catalyst.
The flow rates of both the carrier gas and the carbon precursor must be precisely managed. This ratio affects the concentration of carbon available for deposition on the substrate surface, directly impacting the growth rate and the potential for forming unwanted multi-layer patches.
Chamber and Substrate Materials
The materials used for the reaction chamber and the substrate are not passive components; they are active parts of the physical environment.
The chamber itself must be made of a material, like quartz, that can withstand high temperatures without reacting or outgassing impurities that could contaminate the graphene.
The substrate often doubles as the catalyst. Materials like copper foil or nickel foam are chosen for their ability to catalytically decompose the carbon precursor and provide a surface for the graphene lattice to form.
Understanding the Trade-offs
Optimizing graphene production requires navigating a series of critical trade-offs. There is no single "best" recipe; the ideal conditions depend entirely on the desired outcome.
Quality vs. Speed
Higher temperatures generally accelerate growth, but if not perfectly balanced with precursor flow, can lead to the formation of defects or undesirable multi-layer graphene.
Conversely, very low pressures and temperatures can produce highly uniform, single-layer films but may slow the production rate significantly, making the process less viable for large-scale applications.
Cost vs. Performance
Achieving ultra-low pressures requires expensive high-vacuum pumps, and maintaining temperatures above 1000°C consumes a great deal of energy. These factors drive up the operational cost of producing the highest-purity material.
Using less extreme conditions can lower costs, but often at the expense of uniformity, defect density, or overall material quality.
Process Complexity vs. Purity
While catalysts like copper and nickel are essential, they must be removed from the graphene film after growth. This transfer process can introduce tears, wrinkles, and contamination, degrading the final quality.
Some methods using catalysts like liquid gallium avoid this transfer step, but they introduce their own complexities and material handling challenges.
Optimizing Conditions for Your Goal
Your choice of physical parameters should be directly aligned with the end goal of your synthesis.
- If your primary focus is high-purity, single-layer research: Prioritize a low-pressure CVD system with precise temperature control (near 1050°C) and a high-quality catalytic substrate like copper foil.
- If your primary focus is rapid production for large-area films: Consider higher precursor flow rates and potentially atmospheric pressure systems, but be prepared for trade-offs in uniformity and defect density.
- If your primary focus is minimizing operational cost: Explore lower temperature ranges (around 800-900°C) and catalysts that reduce energy requirements, while accepting a potential decrease in growth rate or crystalline quality.
Mastering these variables transforms graphene synthesis from a complex art into a predictable science.
Summary Table:
| Condition | Key Range/Details | Impact on Graphene Production |
|---|---|---|
| Temperature | 800-1050 °C | Higher temps increase growth speed and quality but raise energy costs and safety risks. |
| Pressure | 1-1500 Pa (LPCVD) | Low pressures reduce unwanted reactions, improving uniformity and single-layer formation. |
| Carrier Gas Flow | Precise control of Ar or H₂ | Affects carbon deposition rate and multi-layer formation; requires balanced ratios. |
| Catalytic Materials | Copper foil, nickel foam | Decompose carbon precursors and support lattice formation; choice influences purity and transfer complexity. |
Unlock the full potential of your graphene synthesis with KINTEK's advanced high-temperature furnace solutions! Leveraging exceptional R&D and in-house manufacturing, we provide diverse laboratories with tailored systems like Muffle, Tube, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems. Our strong deep customization capability ensures precise control over temperature, pressure, and gas flow to meet your unique experimental needs—whether for high-purity research, rapid production, or cost efficiency. Don't let suboptimal conditions hold you back—contact us today to discuss how we can enhance your graphene production process and achieve superior results!
Visual Guide
Related Products
- Custom Made Versatile CVD Tube Furnace Chemical Vapor Deposition CVD Equipment Machine
- Multi Heating Zones CVD Tube Furnace Machine for Chemical Vapor Deposition Equipment
- Inclined Rotary Plasma Enhanced Chemical Deposition PECVD Tube Furnace Machine
- Split Chamber CVD Tube Furnace with Vacuum Station CVD Machine
- RF PECVD System Radio Frequency Plasma Enhanced Chemical Vapor Deposition
People Also Ask
- Why is the tube design important in CVD furnaces? Ensure Uniform Deposition for High-Quality Films
- Which industries and research fields benefit from CVD tube furnace sintering systems for 2D materials? Unlock Next-Gen Tech Innovations
- What role do CVD tube furnace sintering systems play in 2D material synthesis? Enabling High-Quality Atomic Layer Growth
- What temperature ranges can a CVD Tube Furnace achieve with different tube materials? Unlock High-Temp Precision for Your Lab
- What types of atmosphere control does a CVD Tube Furnace support? Master Vacuum and Gas Control for Precision



















