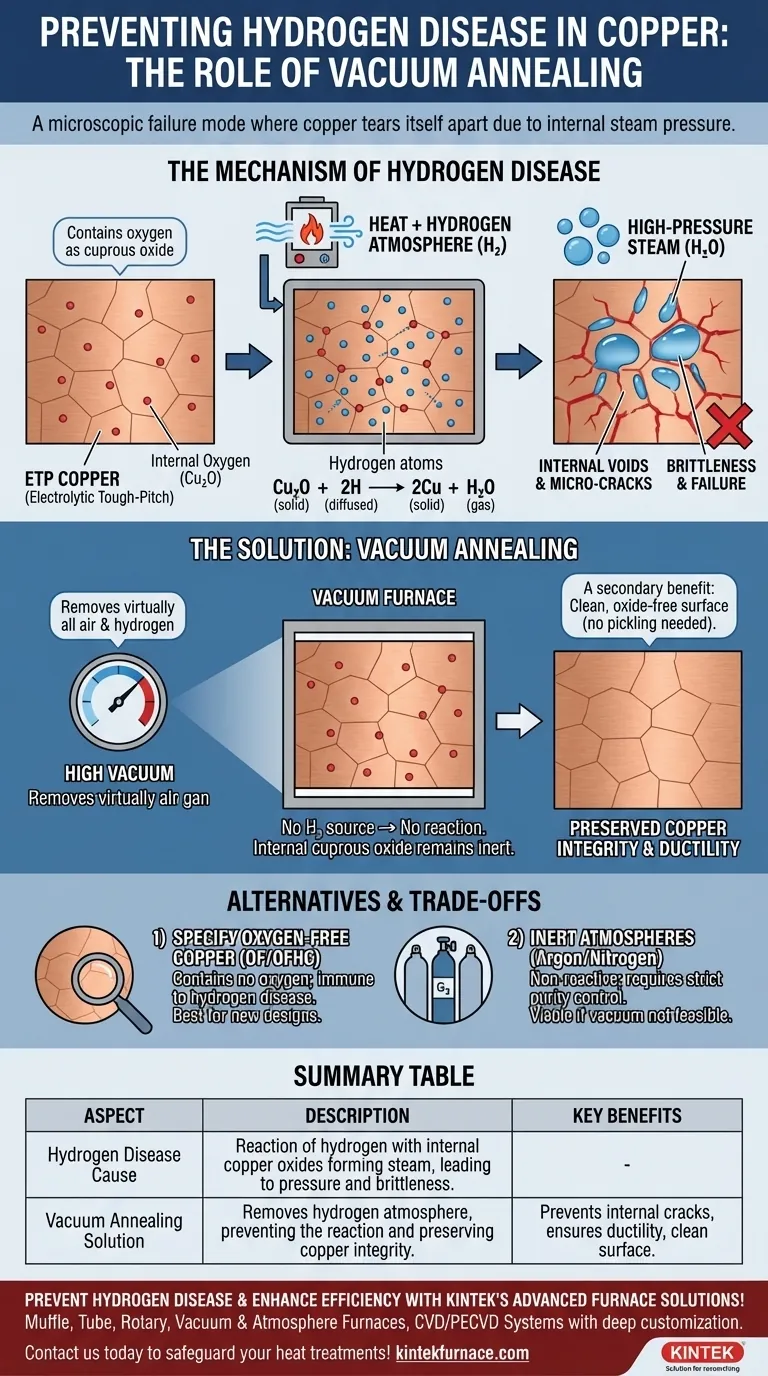
At a microscopic level, hydrogen disease is a catastrophic failure mode where copper essentially tears itself apart from the inside out. This occurs when copper containing oxygen is heated in an atmosphere rich in hydrogen. The tiny hydrogen atoms diffuse into the metal and react with the internal copper oxides to form water vapor (steam), which creates immense internal pressure, leading to micro-cracks and severe brittleness.
The core issue is not with copper itself, but with a specific combination of factors: the presence of oxygen within the copper matrix and the use of a hydrogen-rich atmosphere during heat treatment. Vacuum annealing prevents hydrogen disease by simply removing the reactive gas from the environment.

The Hidden Flaw: Understanding the Mechanism
To prevent a problem, you must first understand its root cause. Hydrogen disease, also known as hydrogen embrittlement in copper, is a classic example of an undesirable chemical reaction occurring within a solid material.
The Ingredients for Failure: Oxygen-Bearing Copper
Most common industrial copper, known as Electrolytic Tough-Pitch (ETP) copper, contains a small but significant amount of oxygen in the form of cuprous oxide (Cu₂O) dispersed within its grain structure.
Under normal conditions, this oxygen is harmless. However, it becomes a critical vulnerability during certain heat treatment processes.
The Catalyst: Heat and a Hydrogen Atmosphere
Annealing is a heat treatment process used to soften copper and increase its ductility, typically after it has been hardened by cold working.
Sometimes, this is done in a reducing atmosphere containing hydrogen (H₂) to prevent surface oxidation and scaling. While this protects the surface, it introduces the agent of failure into the system.
The Chemical Reaction: From Solid Oxide to High-Pressure Steam
When heated, small hydrogen atoms can easily diffuse through the copper's crystal lattice. When they encounter a particle of cuprous oxide, a chemical reaction occurs:
Cu₂O (solid) + 2H (diffused) → 2Cu (solid) + H₂O (gas)
This reaction converts a stable, solid oxide into gaseous water vapor, or steam.
The Result: Internal Voids and Brittleness
The newly formed water molecules are much larger than the hydrogen atoms and become trapped within the copper, typically at the grain boundaries.
As more steam is generated, immense pressure builds in these microscopic pockets. This pressure forces the copper grains apart, creating voids, fissures, and intergranular cracks throughout the material, rendering it extremely brittle and useless for most applications.
Why Vacuum Annealing is the Definitive Solution
Understanding the mechanism makes the solution clear. If the problem is caused by the interaction between internal oxygen and an external hydrogen atmosphere, the most effective solution is to remove the atmosphere.
How It Works: Removing the Reactive Element
Vacuum annealing is the process of heating the material in a high-vacuum chamber. By removing virtually all the air and other gases, there is no external hydrogen source to diffuse into the copper.
Without hydrogen, the chemical reaction that produces high-pressure steam cannot occur. The internal cuprous oxide remains inert, and the material's integrity is preserved.
The Added Benefit: A Clean, Oxide-Free Surface
A secondary benefit of vacuum annealing is that it also prevents surface oxidation. With no oxygen in the chamber, the copper parts emerge from the furnace bright and clean, often eliminating the need for post-treatment acid cleaning or pickling.
Understanding the Trade-offs and Alternatives
While vacuum annealing is highly effective, it's important to consider it within a broader context of material selection and processing costs.
The Upstream Solution: Specifying Oxygen-Free Copper
The most robust way to prevent hydrogen disease is to use a grade of copper that is immune to it from the start.
Oxygen-Free (OF) or Oxygen-Free High-Conductivity (OFHC) copper contains virtually no oxygen. Without the internal cuprous oxide, there is nothing for hydrogen to react with, making these grades completely safe for annealing in any reducing atmosphere.
Alternative Atmospheres: Inert Gases
If vacuum processing is not available or cost-effective, another option is to anneal in a truly inert atmosphere, such as pure argon or nitrogen.
These gases do not react with copper or its internal oxides, preventing both hydrogen disease and surface scaling. However, care must be taken to ensure the purity of the inert gas, as even small hydrogen contaminants can cause issues.
The Cost and Complexity Factor
Vacuum furnaces and the processes associated with them are generally more complex and expensive than atmospheric furnaces. The decision to use vacuum annealing often depends on the cost of failure versus the cost of processing.
Making the Right Choice for Your Application
Selecting the correct approach requires balancing material properties, processing costs, and the final application's reliability requirements.
- If you are working with existing ETP copper parts: Vacuum annealing is the safest and most reliable method to soften the material without risking catastrophic hydrogen disease.
- If you are designing a new component for a high-reliability application: Specify Oxygen-Free (OF/OFHC) copper from the start to completely design out the risk of hydrogen embrittlement.
- If cost is the primary driver and failure risk is low: Annealing ETP copper in a pure inert gas atmosphere can be a viable alternative, but requires strict process control.
Ultimately, preventing hydrogen disease is a matter of making an informed choice to separate the critical ingredients of oxygen, hydrogen, and heat.
Summary Table:
| Aspect | Description |
|---|---|
| Hydrogen Disease Cause | Reaction of hydrogen with internal copper oxides forming steam, leading to pressure and brittleness. |
| Vacuum Annealing Solution | Removes hydrogen atmosphere, preventing the reaction and preserving copper integrity. |
| Key Benefits | Prevents internal cracks, ensures ductility, and provides a clean, oxide-free surface. |
Prevent hydrogen disease and enhance your lab's efficiency with KINTEK's advanced high-temperature furnace solutions! Leveraging exceptional R&D and in-house manufacturing, we offer Muffle, Tube, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems, all with strong deep customization to meet your unique experimental needs. Contact us today to discuss how our tailored solutions can safeguard your copper heat treatments and boost performance!
Visual Guide
Related Products
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Molybdenum Vacuum Heat Treat Furnace
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
People Also Ask
- What are the proper procedures for handling the furnace door and samples in a vacuum furnace? Ensure Process Integrity & Safety
- What are the benefits of vacuum heat treatment? Achieve Superior Metallurgical Control
- What are the general operational features of a vacuum furnace? Achieve Superior Material Purity & Precision
- What role does a high-temperature vacuum heat treatment furnace play in LP-DED? Optimize Alloy Integrity Today
- Why does heating steel rod bundles in a vacuum furnace eliminate heat transfer paths? Enhance Surface Integrity Today



















