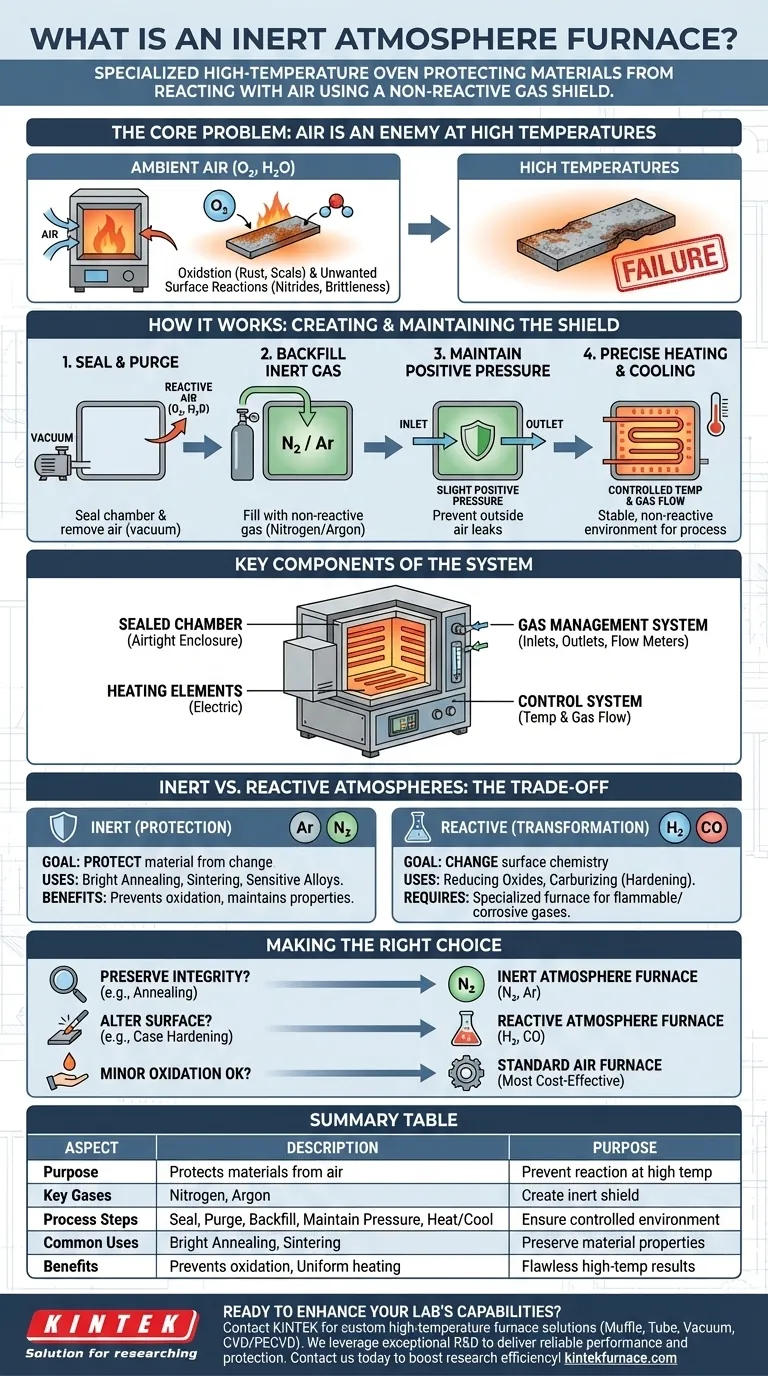
Fundamentally, an inert atmosphere furnace is a specialized high-temperature oven designed to protect materials from reacting with the surrounding air. By creating a vacuum and then filling the chamber with a non-reactive (inert) gas like nitrogen or argon, it prevents processes like oxidation, ensuring the material's chemical and physical properties remain unchanged during heat treatment.
An inert atmosphere furnace isn't just about heating; it's about controlling the chemical environment. While a standard furnace heats materials in open air, an inert furnace creates a protective shield, which is essential for processes where any reaction with oxygen or moisture would compromise the final product.

The Core Problem: Why Air is an Enemy at High Temperatures
Most heat treatment processes fail if performed in ambient air. The gases we breathe, primarily oxygen and water vapor, become highly reactive at elevated temperatures, leading to undesirable chemical changes on a material's surface.
The Universal Threat of Oxidation
Oxidation is the most common issue. When heated, metals and other materials readily react with oxygen to form oxides—what we commonly see as rust or scale. This oxide layer can ruin surface finish, alter dimensions, and degrade the material's electrical or mechanical properties.
Beyond Oxidation: Unwanted Surface Reactions
Air contains more than just oxygen. Nitrogen and moisture can also react with a heated workpiece, leading to the formation of nitrides or other compounds that can make materials brittle or otherwise compromise their integrity.
How an Inert Atmosphere Furnace Works
The furnace's operation is a carefully controlled sequence designed to replace the reactive atmosphere with a pure, non-reactive one.
Step 1: Creating a Controlled Environment
The process begins by sealing the heating chamber. The system then purges the reactive air, often by pulling a vacuum to remove it entirely. Following the purge, the chamber is backfilled with a high-purity inert gas.
Step 2: Maintaining the Inert Shield
To ensure no outside air leaks in and to carry away any residual contaminants, a slight positive pressure is maintained by a continuous, low-volume flow of the inert gas. This gas flows from an inlet, circulates through the chamber, and exits through an outlet or exhaust system.
Step 3: Precise Heating and Cooling
With the protective atmosphere established, the heating elements bring the chamber to the target temperature. Advanced control systems monitor both the temperature (via thermocouples) and the gas flow, ensuring the entire process—heating, soaking, and cooling—occurs within a stable, non-reactive environment.
Key Components of the System
An inert atmosphere furnace is a cohesive system where each part plays a critical role in maintaining the integrity of the process.
The Sealed Chamber
This is the heart of the furnace. It is built from high-temperature-resistant materials and features robust sealing mechanisms, like silicone gaskets or welded flanges, to create an airtight enclosure.
The Gas Management System
This includes the gas inlets and outlets, flow meters, and valves. This system precisely controls the introduction, circulation, and exhausting of the inert gas, which is fundamental to maintaining the purity of the atmosphere.
The Heating and Control Systems
Electric heating elements are most common, as they do not introduce combustion byproducts. A sophisticated controller, linked to thermocouples inside the chamber, manages the temperature profile with high precision, while a separate controller manages the gas flow rates.
Understanding the Trade-offs: Inert vs. Reactive Atmospheres
The term "atmosphere furnace" is broad. The specific gas used defines the furnace's purpose and is the most important decision in process design.
Inert Atmospheres: The Goal is Protection
An inert atmosphere is chemically non-reactive. Its only job is to protect the material from unwanted chemical changes.
Gases like argon (Ar) and nitrogen (N₂) are the most common choices. They are ideal for processes like bright annealing, sintering, and heat-treating sensitive alloys where the goal is to preserve the material's surface and bulk properties perfectly.
Reactive Atmospheres: The Goal is Transformation
In contrast, a reactive atmosphere is used to intentionally change the surface chemistry of a material. This requires a furnace designed to handle flammable or corrosive gases.
Examples include using hydrogen (H₂) to actively reduce surface oxides or using carbon-rich gases (like carbon monoxide) for carburizing to harden the surface of steel. This is a deliberate chemical process, not just protective heating.
Making the Right Choice for Your Process
Selecting the right furnace and atmosphere depends entirely on the desired outcome for your material.
- If your primary focus is preserving material integrity (e.g., annealing copper or sintering powdered metals): You need a true inert atmosphere furnace that uses high-purity nitrogen or argon to prevent any surface reaction.
- If your primary focus is altering a material's surface chemistry (e.g., case hardening steel): You need a reactive atmosphere furnace specifically designed to handle gases like hydrogen, ammonia, or carbon monoxide safely.
- If your primary focus is simple heat treatment where minor oxidation is acceptable or can be removed later: A standard, less complex air furnace is the most cost-effective and practical solution.
Understanding the specific atmospheric needs of your material is the first step toward achieving flawless results at high temperatures.
Summary Table:
| Aspect | Description |
|---|---|
| Purpose | Protects materials from reacting with air during high-temperature processes |
| Key Gases | Nitrogen, Argon |
| Process Steps | Seal chamber, purge air, backfill with inert gas, maintain positive pressure, heat/cool precisely |
| Common Uses | Bright annealing, sintering, heat-treating sensitive alloys |
| Benefits | Prevents oxidation, maintains material properties, ensures uniform heating |
Ready to enhance your lab's capabilities with a custom inert atmosphere furnace? At KINTEK, we leverage exceptional R&D and in-house manufacturing to provide advanced high-temperature furnace solutions tailored to your unique needs. Our product line includes Muffle, Tube, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems, all backed by strong deep customization capabilities. Whether you're working with sensitive alloys or need precise sintering, we can deliver reliable performance and protection. Contact us today to discuss how our furnaces can solve your high-temperature challenges and boost your research efficiency!
Visual Guide
Related Products
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
People Also Ask
- How does a chemically inert atmosphere function in a furnace? Prevent Oxidation and Ensure Material Purity
- How does nitrogen atmosphere heat treatment improve surface strengthening? Enhance Durability and Performance
- What is nitrogen used for in a furnace? Prevent Oxidation and Control Heat Treatment Quality
- What are the environmental benefits of using inert gases in furnaces? Reduce Waste and Emissions for a Greener Process
- What are the benefits of inert atmosphere heat treating? Prevent Oxidation and Preserve Material Integrity



















