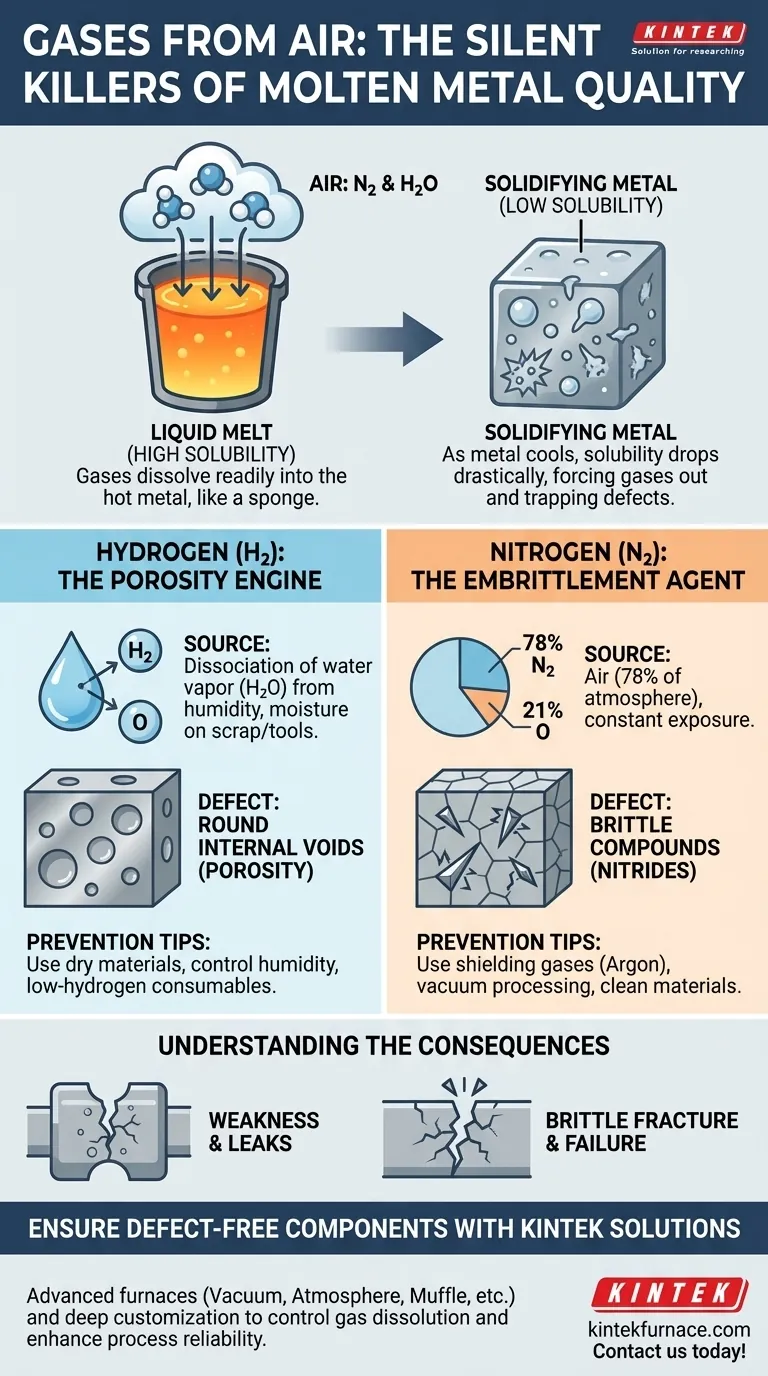
The two gases in the air that cause the most significant defects in molten metal are nitrogen and hydrogen. At the high temperatures of a liquid melt, these gases readily dissolve into the metal, but as it cools and solidifies, their solubility drops drastically, forcing them out of solution and creating damaging internal flaws.
Molten metal acts like a sponge for hydrogen and nitrogen. When the metal solidifies, it can no longer hold these dissolved gases, which then become trapped as bubbles (porosity) or form brittle compounds that compromise the final product's integrity.

The Fundamental Principle: Gas Solubility in Metal
The core issue is a physical law: gases are significantly more soluble in liquid metal than in solid metal. This difference in solubility is the engine that drives defect formation during the casting or welding process.
Think of it like dissolving sugar in water. You can dissolve far more sugar in hot water than in cold water. If you cool a saturated hot sugar solution, the sugar will precipitate out as crystals. Molten metal and dissolved gases behave in a very similar way.
The Problem of High Temperatures
At melting temperatures, the atoms in the metal are loosely arranged and have high energy, leaving more space for small gas atoms like hydrogen and nitrogen to diffuse into the liquid. The surrounding atmosphere provides a nearly limitless supply of these gases.
The Critical Cooling Phase
As the metal cools and begins to solidify, its crystalline structure becomes more rigid and ordered. There is simply no room for the dissolved gas atoms. This sudden drop in solubility forces the gas out of the metal matrix. Because the exterior of the casting or weld solidifies first, this escaping gas becomes trapped within the solidifying metal.
The Two Primary Culprits from the Air
While the air is a mixture of gases, nitrogen and hydrogen are the most problematic due to their atomic size, reactivity, and abundance.
Hydrogen (H): The Porosity Engine
Hydrogen is the smallest atom and can easily diffuse into molten metal. The primary source is not typically gaseous hydrogen but the dissociation of water vapor (H₂O) from humidity in the air or moisture on scrap metal, tools, or fluxes.
At high temperatures, water breaks down, releasing hydrogen to be absorbed by the melt. Upon cooling, this hydrogen is aggressively rejected from the solidifying metal, forming round, internal voids known as hydrogen porosity.
Nitrogen (N₂): The Embrittlement Agent
Nitrogen makes up approximately 78% of the air, creating constant exposure. Like hydrogen, it can cause porosity, but its more insidious effect is its ability to react with the base metal and alloying elements.
In metals like steel, aluminum, and especially titanium, dissolved nitrogen can form hard, brittle compounds called nitrides during cooling. These nitrides act as internal stress points, drastically reducing the material's ductility and toughness, a phenomenon known as embrittlement.
Understanding the Consequences
The defects caused by dissolved gases are not merely cosmetic; they directly impact the mechanical performance and reliability of the final component.
Porosity: The "Swiss Cheese" Effect
Gas porosity creates a network of internal voids. This reduces the cross-sectional area of the component, making it weaker and less dense. These smooth, spherical voids also act as stress concentrators, providing an easy initiation point for cracks to form and grow under load, leading to premature failure.
Embrittlement: Nitrides and Hydrides
Unlike empty voids, nitrides (and less commonly, hydrides) are physical particles embedded within the metal's grain structure. These hard, ceramic-like inclusions disrupt the continuity of the metallic lattice.
They prevent the metal from deforming plastically under stress, causing it to fracture in a brittle manner with little or no warning. This is particularly dangerous in applications requiring toughness and impact resistance.
Making the Right Choice for Your Process
Controlling the atmosphere and raw materials is not an optional step; it is fundamental to producing sound metallic components. Your specific focus will depend on the primary failure mode you need to prevent.
- If your primary focus is preventing structural weakness and leaks: Your main goal is to minimize hydrogen absorption to reduce porosity. This means ensuring all materials are dry and controlling humidity.
- If your primary focus is ensuring ductility and impact strength: You must control nitrogen exposure, especially in sensitive alloys. This may require using shielding gases (like argon) or vacuum processing.
- If your primary focus is overall quality in high-performance alloys: You must aggressively control both hydrogen and nitrogen through a combination of clean raw materials, vacuum or inert gas protection, and specific melt treatments.
Ultimately, mastering the interaction between the atmosphere and the melt is essential for translating a good design into a reliable, high-performance product.
Summary Table:
| Gas | Primary Source | Main Defects Caused | Prevention Tips |
|---|---|---|---|
| Hydrogen | Water vapor, humidity | Porosity (voids) | Use dry materials, control humidity |
| Nitrogen | Air (78% of atmosphere) | Embrittlement (nitrides) | Use shielding gases, vacuum processing |
Ensure your metal components are defect-free with KINTEK's advanced furnace solutions! Leveraging exceptional R&D and in-house manufacturing, we provide diverse laboratories with high-temperature furnaces like Muffle, Tube, Rotary, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems. Our deep customization capability precisely meets your unique experimental needs to control gas dissolution and prevent defects. Contact us today to enhance your process reliability and product quality!
Visual Guide
Related Products
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- Vacuum Induction Melting Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
People Also Ask
- How is the sealing performance of an experimental box type atmosphere furnace enhanced? Boost Purity with Advanced Sealing Systems
- How do atmosphere furnaces contribute to ceramic manufacturing? Enhance Purity and Performance
- How does a batch type controlled atmosphere furnace operate? Master Precision Heat Treatment for Superior Materials
- What are the development prospects of atmosphere box furnaces in the aerospace industry? Unlock Advanced Material Processing for Aerospace Innovation
- What are some specific applications of atmosphere furnaces in the ceramics industry? Enhance Purity and Performance



















