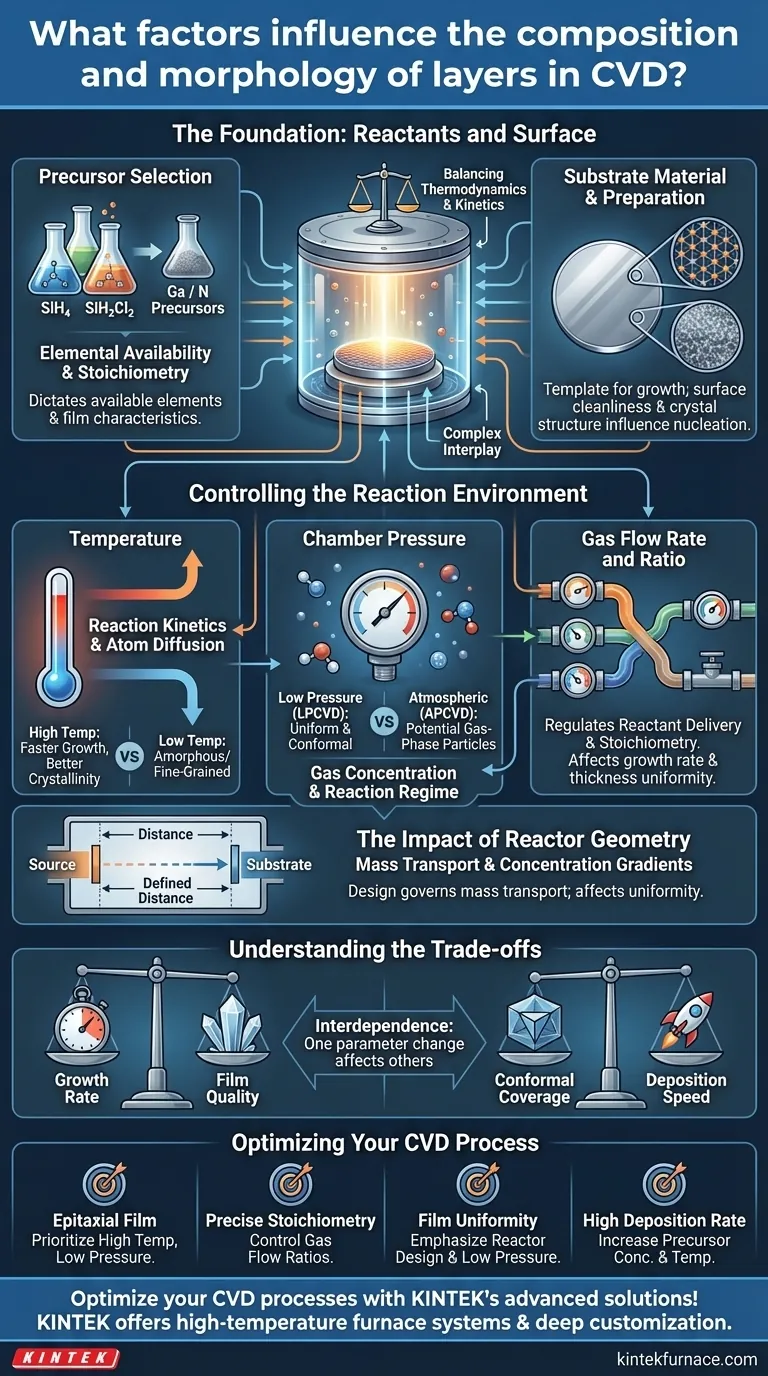
In Chemical Vapor Deposition (CVD), the final composition and physical structure (morphology) of the deposited layer are not accidental. They are the direct result of a complex interplay between several key process parameters, including the chemical precursors and substrate, temperature, pressure, gas flow rates and ratios, and the physical geometry of the reactor.
The core challenge of CVD is not just knowing which parameters matter, but understanding how they interact. Achieving a desired film requires balancing the thermodynamics of the chemical reaction with the kinetics of mass transport within the reactor.

The Foundation: Reactants and Surface
The starting materials and the surface you deposit onto are the fundamental building blocks of your entire process.
Precursor Selection
The choice of precursor chemicals is the most fundamental decision, as it dictates which elements are available for deposition. Precursors must be sufficiently volatile to be transported in a gas phase and must decompose at a temperature compatible with the substrate.
For example, depositing silicon (Si) can be done using silane (SiH₄) at lower temperatures or dichlorosilane (SiH₂Cl₂) at higher temperatures, each yielding different film characteristics and byproducts.
Substrate Material and Preparation
The substrate is not a passive holder; it is the template for film growth. Its chemical nature, crystal structure (crystallinity), and surface cleanliness directly influence the initial nucleation and subsequent growth of the film.
A perfectly clean, single-crystal silicon wafer will promote epitaxial (highly ordered) growth, while an amorphous or poorly cleaned surface will likely result in a polycrystalline or amorphous film.
Controlling the Reaction Environment
Once the chemicals and substrate are chosen, the reactor environment dictates how the film forms. These parameters control the energy and supply of reactants.
The Critical Role of Temperature
Temperature is the primary driver of the CVD reaction. It supplies the activation energy needed for precursors to decompose on the substrate surface and for deposited atoms to diffuse and find their ideal positions in the crystal lattice.
- Higher temperatures generally lead to faster growth rates and better crystallinity as atoms have more energy to move.
- Lower temperatures can result in amorphous or fine-grained polycrystalline films because atom mobility is limited.
Chamber Pressure's Influence
Chamber pressure controls the concentration of gas molecules and their mean free path (the average distance a molecule travels before colliding with another).
At low pressures (LPCVD), molecules travel longer distances and reactions are more likely to occur on the substrate surface. This "surface-reaction-limited" regime often produces highly uniform and conformal films.
At atmospheric pressures (APCVD), frequent gas-phase collisions can lead to the formation of particles in the gas stream, which can then fall onto the substrate, degrading film quality.
Gas Flow Rate and Ratio
The carrier gas flow rate determines how quickly reactants are delivered to the substrate and how fast byproducts are removed. This directly impacts the growth rate.
The ratio of different source gases is paramount for controlling the film's stoichiometry, or its elemental composition. For instance, in depositing gallium nitride (GaN), the ratio of the gallium precursor to the nitrogen precursor is meticulously controlled to achieve the desired material properties.
The Impact of Reactor Geometry
The physical design of the reactor, including the distance between the gas source and the substrate, governs the mass transport of reactants. This geometry creates concentration gradients that influence film uniformity and growth rate.
A shorter source-substrate distance can increase the deposition rate but may compromise the film's thickness uniformity across the wafer.
Understanding the Trade-offs
Optimizing a CVD process is an exercise in balancing competing factors. Changing one parameter will inevitably affect others.
Growth Rate vs. Film Quality
This is the most common trade-off. Aggressively increasing temperature or precursor concentration to achieve a high growth rate often comes at the cost of quality. It can introduce defects, stress, or a rougher surface morphology.
Conformal Coverage vs. Deposition Speed
Achieving excellent conformality—the ability of a film to evenly coat complex, three-dimensional topographies—often requires operating in a surface-reaction-limited regime (e.g., LPCVD). This regime is typically slower than mass-transport-limited processes.
The Interdependence of Parameters
No parameter exists in a vacuum. An increase in temperature might require an adjustment in pressure to prevent unwanted gas-phase reactions. Similarly, changing the gas flow rate can alter the optimal temperature profile within the reactor. Effective process development involves co-optimizing these interdependent variables.
Optimizing Your CVD Process
Your approach to tuning these parameters should be dictated by your end goal for the deposited film.
- If your primary focus is a high-quality, single-crystal (epitaxial) film: Prioritize high temperatures to ensure atom mobility and a low pressure, surface-reaction-limited regime for controlled, layer-by-layer growth.
- If your primary focus is precise stoichiometry (e.g., for compound semiconductors): Meticulously control the partial pressures and flow rate ratios of your reactive source gases.
- If your primary focus is film uniformity across a large area: Emphasize reactor design, gas flow dynamics, and often a lower pressure to ensure every part of the substrate receives a similar flux of reactants.
- If your primary focus is a high deposition rate: Increase precursor concentration and temperature, but be prepared to manage the potential for reduced film quality or gas-phase particle formation.
Mastering CVD comes from systematically understanding how each of these levers influences the delicate balance between chemical reaction and physical transport.
Summary Table:
| Factor | Influence on Composition | Influence on Morphology |
|---|---|---|
| Precursor Selection | Determines elemental availability and stoichiometry | Affects nucleation and growth characteristics |
| Substrate Material | Impacts chemical bonding and initial nucleation | Influences epitaxial vs. polycrystalline growth |
| Temperature | Controls reaction kinetics and atom diffusion | Higher temps improve crystallinity; lower temps may cause amorphous films |
| Pressure | Affects gas concentration and reaction regime (e.g., LPCVD for uniformity) | Influences film uniformity and conformality |
| Gas Flow Rate/Ratio | Regulates reactant delivery and stoichiometry | Affects growth rate and thickness uniformity |
| Reactor Geometry | Governs mass transport and concentration gradients | Impacts deposition rate and film uniformity across the substrate |
Optimize your CVD processes with KINTEK's advanced solutions! Leveraging exceptional R&D and in-house manufacturing, we provide diverse laboratories with high-temperature furnace systems like Muffle, Tube, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems. Our strong deep customization capability ensures precise alignment with your unique experimental needs, helping you achieve superior film composition and morphology. Contact us today to discuss how we can enhance your research and production outcomes!
Visual Guide
Related Products
- Inclined Rotary Plasma Enhanced Chemical Deposition PECVD Tube Furnace Machine
- RF PECVD System Radio Frequency Plasma Enhanced Chemical Vapor Deposition
- Inclined Rotary Plasma Enhanced Chemical Deposition PECVD Tube Furnace Machine
- Custom Made Versatile CVD Tube Furnace Chemical Vapor Deposition CVD Equipment Machine
- Multi Heating Zones CVD Tube Furnace Machine for Chemical Vapor Deposition Equipment
People Also Ask
- How does a PECVD system contribute to (n)poly-Si layers? High-Throughput In-Situ Doping Explained
- Why Use PECVD for Monolithic Integrated Chip Isolation Layers? Protect Your Thermal Budget with High-Quality SiO2
- What environments does a PECVD system provide for silicon nanowires? Optimize Growth with Precise Thermal Control
- How does a CVD system ensure the quality of carbon layers? Achieving Nanometer Precision with KINTEK
- What are the future trends in CVD technology? AI, Sustainability, and Advanced Materials



















