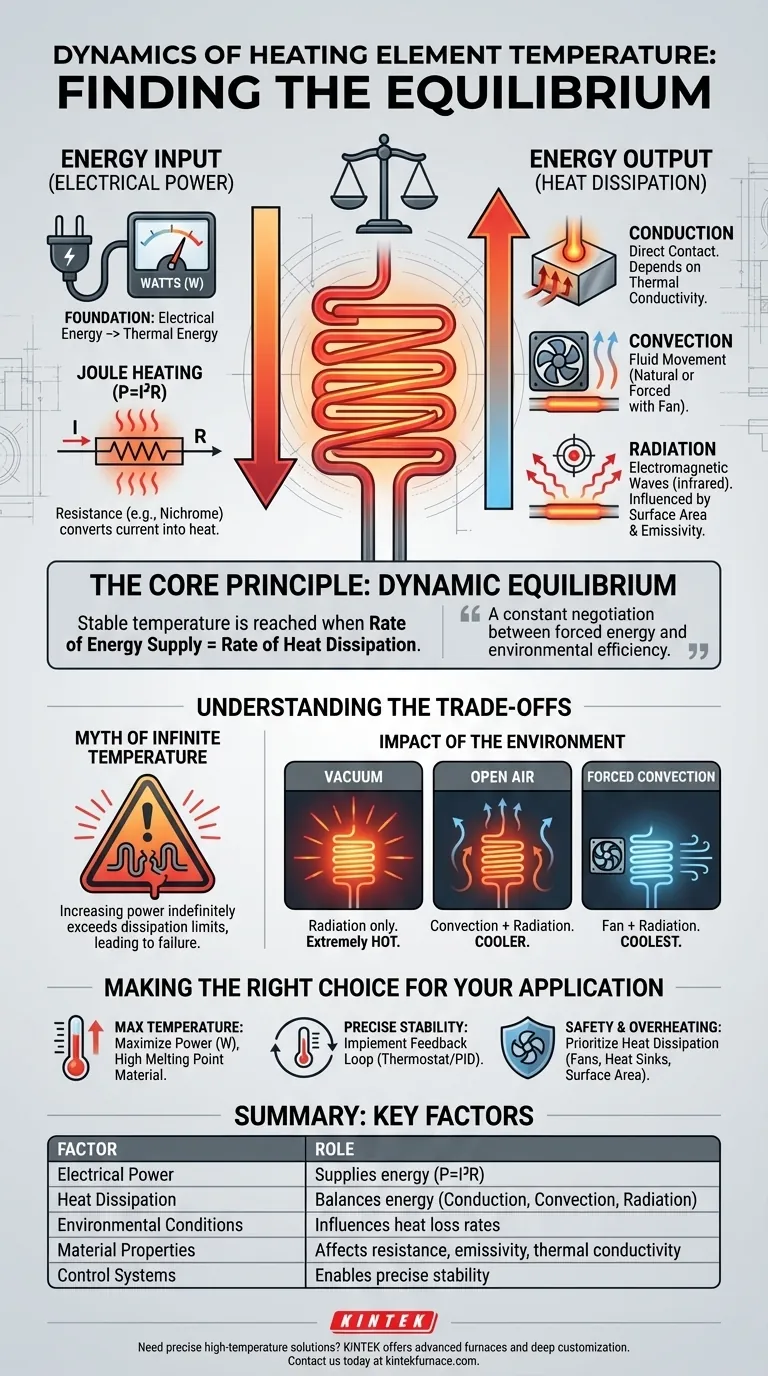
The temperature of a heating element is a direct result of a dynamic equilibrium. This stable temperature is reached when the rate of energy being supplied to the element (electrical power) becomes equal to the rate at which that energy is transferred away from the element as heat into its surroundings. Without this balance, the element would either heat up indefinitely until it failed or never reach a stable operating temperature.
A heating element's temperature isn't determined by power alone. It is a constant negotiation between the energy you force into it and the efficiency with which its environment can carry that energy away.

The Input Side: Electrical Power as Heat
The energy supplied to a heating system is the foundation of its temperature. This input is almost always in the form of electrical energy, which is converted into thermal energy.
The Role of Power (Watts)
The rate of energy supply is measured in watts (W). One watt is equivalent to one joule of energy supplied per second.
Increasing the wattage directly increases the rate at which the element's internal energy rises, causing its temperature to climb faster and reach a higher equilibrium point.
The Principle of Joule Heating
This energy conversion happens because of the element's electrical resistance (R). As current (I) flows through the resistive material, electrical energy is converted into heat.
This phenomenon, known as Joule heating, is described by the formula P = I²R. Materials like Nichrome are used for heating elements because they have high resistance and can tolerate very high temperatures without melting or oxidizing.
The Output Side: How Heat Escapes
The temperature an element can reach is ultimately limited by its ability to dissipate heat into its surroundings. This occurs through three distinct mechanisms of heat transfer.
Conduction
Conduction is the transfer of heat through direct physical contact. Heat flows from the hotter element to any cooler object it touches, such as mounting brackets, ceramic insulators, or a metal pot.
The effectiveness of conduction depends on the thermal conductivity of the materials in contact. A copper heat sink will pull heat away far more effectively than a plastic mount.
Convection
Convection is the transfer of heat through the movement of fluids (like air or water). As the fluid near the element heats up, it becomes less dense and rises, allowing cooler fluid to take its place and absorb more heat.
This process can be passive (natural convection) or active (forced convection), such as when a fan blows air across the element. Forced convection dramatically increases the rate of heat transfer, leading to a lower element temperature for the same power input.
Radiation
Radiation is the transfer of heat via electromagnetic waves (specifically, infrared radiation). All objects above absolute zero emit thermal radiation.
The rate of radiative heat transfer is heavily influenced by the element's surface area and its emissivity—a measure of how effectively a surface radiates energy. A matte black surface has a high emissivity and radiates heat well, while a shiny, polished surface has low emissivity and radiates poorly.
Understanding the Trade-offs
Simply looking at power input or heat transfer in isolation is a common mistake. The final temperature is always a result of the interplay between these factors.
The Myth of Infinite Temperature
You cannot make an element infinitely hot just by increasing power. At a certain point, the rate of energy input will exceed the maximum possible rate of heat dissipation.
When this happens, the temperature will rise uncontrollably until the element melts or burns out. This is why proper system design, including ventilation and material choice, is critical.
The Impact of the Environment
The same heating element operating at the same power will have vastly different temperatures in different environments.
An element in a vacuum can only cool by radiation, so it will get extremely hot. The same element in open air will be cooler due to convection, and even cooler still if a fan is actively blowing air across it.
Making the Right Choice for Your Application
Your approach to managing temperature depends entirely on your project's goal.
- If your primary focus is reaching a maximum temperature: Maximize power input (watts) while selecting an element material with a very high melting point and low reactivity.
- If your primary focus is maintaining a precise, stable temperature: Implement a feedback loop, such as a thermostat or PID controller, that modulates power input to perfectly balance the system's heat loss.
- If your primary focus is safety and preventing overheating: Prioritize increasing the rate of heat dissipation through forced convection (fans), conduction (heat sinks), or designing for a larger, high-emissivity surface area.
Ultimately, mastering temperature control lies in managing both the energy you introduce and the pathways you provide for it to escape.
Summary Table:
| Factor | Role in Temperature Determination |
|---|---|
| Electrical Power (Watts) | Supplies energy input via Joule heating (P = I²R) |
| Heat Dissipation | Balances energy through conduction, convection, and radiation |
| Environmental Conditions | Influences heat loss rates (e.g., vacuum vs. air) |
| Material Properties | Affects resistance, emissivity, and thermal conductivity |
| Control Systems | Enables precise temperature stability with feedback loops |
Need precise high-temperature solutions for your lab? KINTEK leverages exceptional R&D and in-house manufacturing to provide advanced furnaces like Muffle, Tube, Rotary, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems. With strong deep customization capabilities, we tailor solutions to meet your unique experimental needs. Contact us today to enhance your thermal control and efficiency!
Visual Guide
Related Products
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- Multi Zone Laboratory Quartz Tube Furnace Tubular Furnace
People Also Ask
- What role does a muffle furnace play in analyzing the combustion residues? Optimize Your Composite Char Analysis
- What is the primary role of a muffle furnace in the annealing process of AlCrTiVNbx alloys? Enhance Alloy Strength
- Why is a muffle furnace used to determine the ash content of biochar? Master Your Material Purity Analysis
- How does a muffle furnace contribute to kaolin-modified biochar? Optimize Pyrolysis & Mineral Integration
- Why are precision stirring and drying equipment necessary for photocatalytic materials? Master Microstructure Control



















