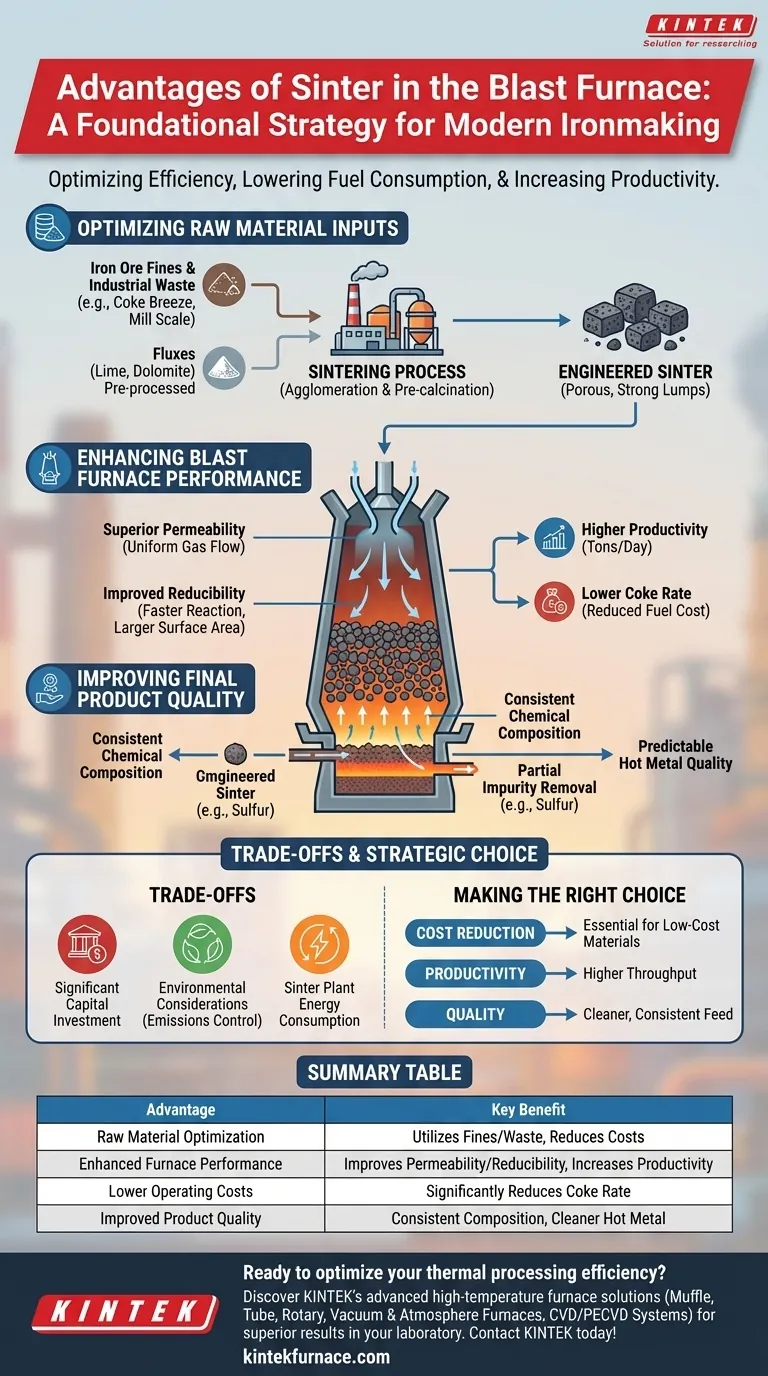
In short, using sinter is a foundational strategy for modern ironmaking. It allows a blast furnace to operate with higher efficiency, lower fuel consumption, and greater productivity by transforming low-cost raw materials like iron ore fines and industrial waste into a superior, engineered furnace feed.
The core advantage of sinter is not merely that it allows for the use of fine particles. It is a purpose-built material designed to optimize the physical and chemical conditions inside the blast furnace, leading to significant economic and operational gains that are impossible to achieve with raw ore alone.

Optimizing Raw Material Inputs
The use of sinter fundamentally changes the economics of raw material procurement and preparation for a blast furnace.
Utilization of Fines and Waste
Raw iron ore contains a significant fraction of fine particles. Feeding these directly into a blast furnace would clog the system, blocking the flow of critical hot gases and halting production.
Sintering solves this by agglomerating, or binding, these fines into larger, porous lumps. This process also allows for the recycling of valuable iron-bearing wastes from other plant operations, such as coke breeze, mill scale, and flue dust, turning waste streams into a valuable input.
Pre-processing of Fluxes
Sinter allows for the incorporation of fluxing agents like lime and dolomite directly into the agglomerated material.
This is highly efficient because the chemical reactions needed to break down these fluxes (calcination) occur in the sinter plant, not in the blast furnace. This pre-processing saves a significant amount of energy inside the furnace, which can then be used for the primary task of reducing iron oxide.
Enhancing Blast Furnace Performance
A blast furnace fed with a high-quality sinter burden operates more smoothly and efficiently.
Superior Permeability
Sinter is engineered to be both strong and porous. This structure creates a permeable bed inside the furnace, allowing hot reducing gases to flow uniformly through the entire stack of materials.
This even gas flow is essential for efficient heat transfer and ensures that chemical reactions occur consistently throughout the furnace, preventing channeling and improving overall stability.
Improved Reducibility
The porous structure of sinter exposes a greater surface area of iron oxide to the reducing gases (primarily carbon monoxide). This property, known as reducibility, means the iron oxides are converted to metallic iron faster and more completely.
Better reducibility allows the furnace to operate more intensely and efficiently.
Higher Productivity and Lower Coke Rate
The combined effects of excellent permeability and high reducibility directly lead to major performance gains. The furnace can process material more quickly, increasing productivity (tons of hot metal per day).
Simultaneously, the improved thermal and chemical efficiency means less fuel is required to produce each ton of iron. This results in a significant reduction in the coke rate, which is often the single largest operating cost for a blast furnace.
Improving Final Product Quality
The quality of the material going into the furnace directly dictates the quality of the product coming out.
Consistent Chemical Composition
The sintering process involves extensive blending and controlled proportioning of various raw materials. This produces a furnace feed with a highly consistent and predictable chemical makeup.
This consistency eliminates the variability inherent in using raw ores, giving operators much tighter control over the blast furnace process.
Partial Removal of Impurities
The high temperatures of the sintering process (around 1300-1400°C) help to drive off some volatile impurities, such as sulfur, from the raw materials.
This pre-cleaning step results in a cleaner feed material, which in turn reduces the impurity load in the final hot metal.
Predictable Hot Metal Quality
When the blast furnace is fed a consistent, clean, and highly reducible material, the output becomes equally predictable. This leads to improved quality and consistency of the hot metal, which simplifies and reduces costs in the subsequent steelmaking steps.
Understanding the Trade-offs
While highly advantageous, the decision to use sinter is not without its own set of complexities and costs.
Significant Capital Investment
A sintering plant is a massive piece of industrial equipment. The capital expenditure to build one is substantial, and it requires significant land area and supporting infrastructure.
Environmental Considerations
The sintering process itself generates airborne emissions, including sulfur oxides (SOx), nitrogen oxides (NOx), and particulate dust. Modern sinter plants require extensive and costly gas cleaning and pollution control systems to meet environmental regulations.
Energy Consumption
While sinter saves a tremendous amount of energy inside the blast furnace, the sintering process itself is energy-intensive, requiring fuel (typically coke breeze) to generate the necessary heat for agglomeration. This represents a strategic shift of energy consumption from the furnace to the sinter plant.
Making the Right Choice for Your Goal
The use of sinter is a strategic decision based on optimizing the entire ironmaking value chain.
- If your primary focus is cost reduction: Sinter is essential for using low-cost iron ore fines and metallurgical waste, while also dramatically lowering the coke rate.
- If your primary focus is productivity and throughput: The superior permeability and reducibility of sinter directly translate to a higher production rate from the blast furnace.
- If your primary focus is hot metal quality: Sinter provides a chemically uniform and cleaner furnace feed, leading to more predictable and higher-quality hot metal for the steel plant.
Ultimately, integrating sinter into blast furnace operations is a key strategy for transforming lower-grade materials into a high-performance input that maximizes overall plant efficiency.
Summary Table:
| Advantage | Key Benefit |
|---|---|
| Raw Material Optimization | Utilizes iron ore fines and industrial waste, reducing costs. |
| Enhanced Furnace Performance | Improves permeability and reducibility, increasing productivity. |
| Lower Operating Costs | Significantly reduces the coke rate, the largest operating expense. |
| Improved Product Quality | Provides consistent chemical composition and cleaner hot metal. |
Ready to optimize your thermal processing efficiency? Just as sinter revolutionizes blast furnace operations, KINTEK's advanced high-temperature furnace solutions can transform your laboratory's capabilities. Leveraging exceptional R&D and in-house manufacturing, we provide diverse laboratories with advanced solutions like Muffle, Tube, Rotary, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems. Our strong deep customization capability ensures we can precisely meet your unique experimental requirements, helping you achieve superior results, reduce costs, and increase productivity. Contact KINTEL today to discuss how our furnaces can be engineered for your success!
Visual Guide
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Dental Porcelain Zirconia Sintering Ceramic Vacuum Press Furnace
- Spark Plasma Sintering SPS Furnace
People Also Ask
- What role does a laboratory tube furnace perform during the carbonization of LCNSs? Achieve 83.8% Efficiency
- What are the key operational considerations when using a lab tube furnace? Master Temperature, Atmosphere & Safety
- Why is a tube furnace utilized for the heat treatment of S/C composite cathode materials? Optimize Battery Stability
- What is an example of a material prepared using a tube furnace? Master Precise Material Synthesis
- How is a high-temperature tube furnace utilized in the synthesis of MoO2/MWCNTs nanocomposites? Precision Guide



















