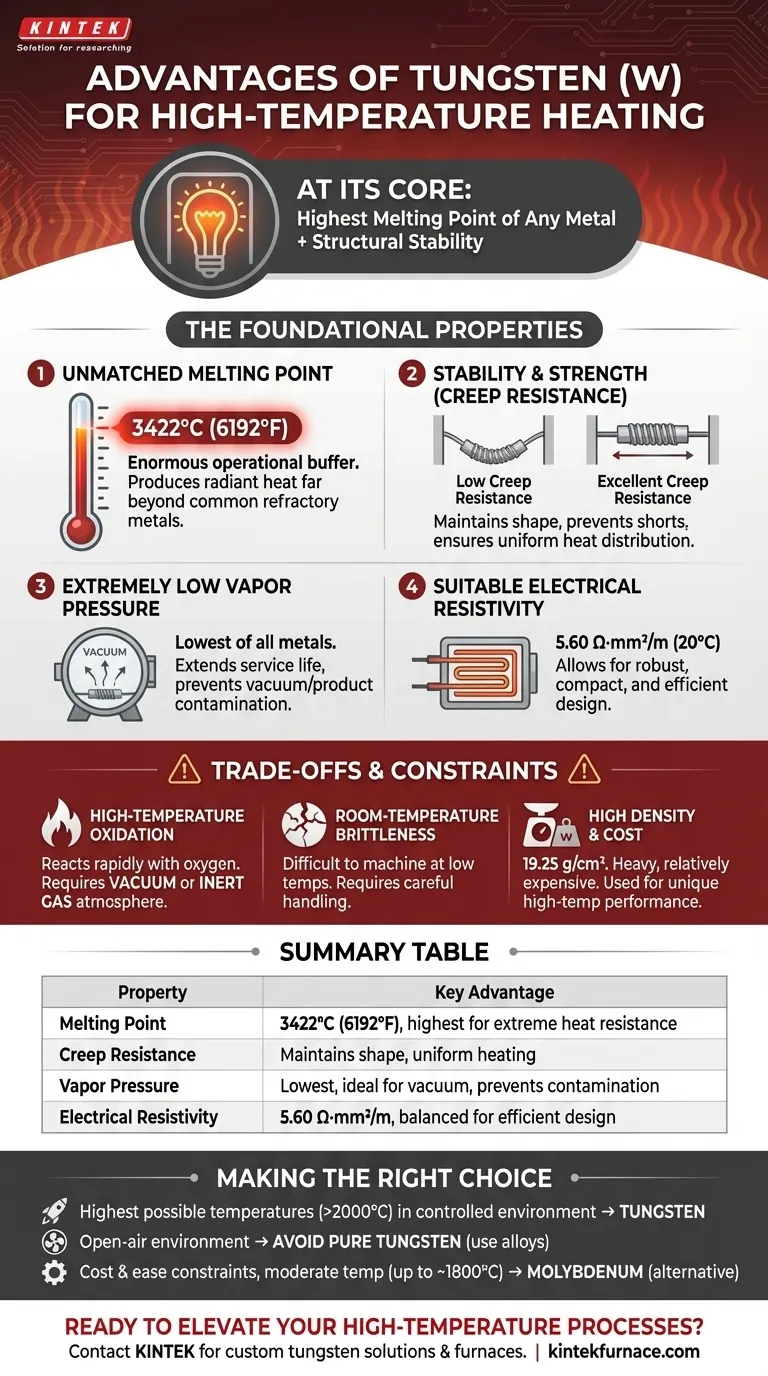
At its core, tungsten's advantage in high-temperature heating applications stems from one primary characteristic: the highest melting point of any known metal. This, combined with its structural stability under extreme heat, allows it to function reliably at temperatures where most other materials would have already melted or deformed.
While its record-setting melting point is its most famous trait, tungsten's true value lies in a unique combination of high-temperature strength, low vapor pressure, and suitable electrical resistivity. These factors allow it to function as a durable heating element where other materials would simply fail, deform, or evaporate.

The Foundational Properties of Tungsten
To understand why tungsten is the material of choice for applications like vacuum furnaces, you must look beyond its melting point and consider the physics of a heating element.
Unmatched Melting Point
The single most important property is its melting point of 3422°C (6192°F). This provides an enormous operational buffer.
Heating elements function by resisting the flow of electricity, which generates heat. Tungsten's ability to remain solid at incredibly high temperatures allows it to produce radiant heat far beyond the capacity of common refractory metals like molybdenum or tantalum.
Stability and Strength Under Heat
A material's melting point is irrelevant if it cannot maintain its shape. At high temperatures, metals tend to sag or deform under their own weight, a phenomenon known as creep.
Tungsten exhibits excellent creep resistance, ensuring the heating element maintains its intended shape. This prevents short-circuiting and ensures uniform heat distribution within the furnace or chamber.
Extremely Low Vapor Pressure
In vacuum applications, a material's tendency to evaporate (its vapor pressure) is critical. Tungsten has the lowest vapor pressure of all metals.
This means it does not readily turn into a gas, even at thousands of degrees in a vacuum. This is vital for two reasons: it extends the service life of the heating element and prevents the tungsten atoms from contaminating the vacuum environment or the product inside.
Suitable Electrical Resistivity
Tungsten's electrical resistivity of 5.60 Ω·mm²/m at 20°C is in a practical "goldilocks" zone.
If resistivity were too low, you would need an impractically long and thin wire to generate sufficient heat, making the element fragile. If it were too high, you might require excessive voltage. Tungsten's resistivity allows for the design of robust, compact, and efficient heating elements.
Understanding the Trade-offs and Constraints
No material is perfect. Selecting tungsten requires you to design around its specific limitations, which are just as important to understand as its strengths.
High-Temperature Oxidation
This is tungsten's most significant drawback. It reacts rapidly with oxygen at high temperatures and will literally burn away in open air.
For this reason, tungsten heating elements can only be operated in a vacuum or a protective, inert gas atmosphere (such as argon or nitrogen).
Room-Temperature Brittleness
Tungsten is famously brittle at and below room temperature. This can make it difficult to machine, form, and install without specialized techniques.
Once it reaches its operating temperature, it becomes more ductile. However, careful handling during manufacturing and maintenance is essential to prevent fractures.
High Density and Cost
With a density of 19.25 g/cm³, tungsten is one of the densest elements, comparable to gold and platinum.
This makes it a heavy material, which can be a design consideration for large heating assemblies. It is also a relatively expensive refractory metal, meaning it is specified when its unique high-temperature performance is a strict requirement.
Making the Right Choice for Your Goal
When selecting a material, your decision should be driven by the specific demands of your application.
- If your primary focus is reaching the highest possible operating temperatures (above 2000°C) in a controlled environment: Tungsten is often the only viable choice due to its unparalleled melting point and low vapor pressure.
- If your application operates in an open-air environment: You must avoid pure tungsten and instead consider iron-chromium-aluminum (FeCrAl) or nickel-chromium (NiCr) alloys, which form a protective oxide layer.
- If cost and ease of fabrication are major constraints for a moderate-temperature vacuum application (up to ~1800°C): Molybdenum can be a more suitable alternative, offering a balance of properties at a lower price point than tungsten.
Ultimately, understanding these fundamental properties and constraints allows you to leverage tungsten's exceptional capabilities while avoiding its operational pitfalls.
Summary Table:
| Property | Key Advantage |
|---|---|
| Melting Point | 3422°C (6192°F), highest of all metals for extreme heat resistance |
| Creep Resistance | Maintains shape under high temperatures, ensuring uniform heating |
| Vapor Pressure | Lowest among metals, ideal for vacuum environments to prevent contamination |
| Electrical Resistivity | 5.60 Ω·mm²/m, balanced for efficient, compact heating element design |
Ready to elevate your high-temperature processes? Leveraging exceptional R&D and in-house manufacturing, KINTEK provides diverse laboratories with advanced solutions like Muffle, Tube, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems. Our strong deep customization capability ensures we precisely meet your unique experimental requirements. Contact us today to discuss how our tungsten-based heating elements can enhance your efficiency and reliability!
Visual Guide
Related Products
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Silicon Carbide SiC Thermal Heating Elements for Electric Furnace
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- Molybdenum Vacuum Heat Treat Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
People Also Ask
- What is the mechanism of a vacuum sintering furnace for AlCoCrFeNi2.1 + Y2O3? Optimize Your High-Entropy Alloy Processing
- Why is a vacuum environment essential for sintering Titanium? Ensure High Purity and Eliminate Brittleness
- What is the role of sintering or vacuum induction furnaces in battery regeneration? Optimize Cathode Recovery
- Why is a high-vacuum environment necessary in copper slag impoverishment? Maximize Your Matte Separation Efficiency
- Why must sintering equipment maintain a high vacuum for high-entropy carbides? Ensure Phase Purity and Peak Density



















