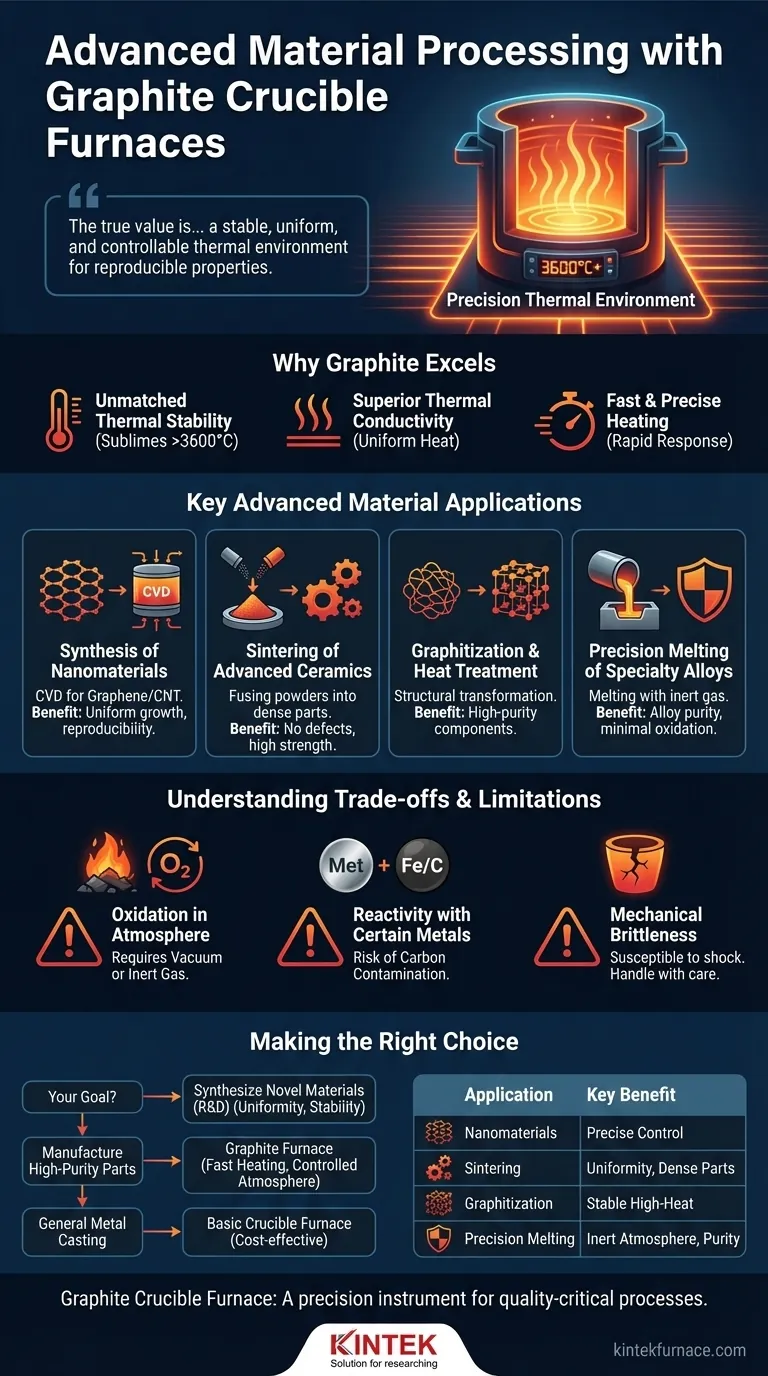
In advanced material processing, graphite crucible furnaces are indispensable for the synthesis of nanomaterials like graphene and carbon nanotubes, as well as for critical processes such as graphitization and the sintering of high-performance ceramics and powders. Their utility stems directly from their ability to achieve and maintain exceptionally high temperatures with remarkable precision and uniformity.
The true value of a graphite crucible furnace isn't just its ability to get hot, but its capacity to provide a stable, uniform, and controllable thermal environment. This precision is what enables the creation of advanced materials with specific, reproducible properties.

The Foundation: Why Graphite Excels at High Temperatures
To understand the applications, you must first understand the material itself. Graphite's unique properties make it an ideal container for high-temperature material processing.
Unmatched Thermal Stability
Unlike most materials, graphite does not have a melting point at atmospheric pressure. Instead, it sublimes at temperatures exceeding 3,600°C (6,500°F), making it exceptionally stable for processes that run far hotter than what conventional metal crucibles can withstand.
Superior Thermal Conductivity
Graphite possesses excellent thermal conductivity. This ensures that heat from the furnace element is transferred evenly and efficiently throughout the crucible, eliminating hot or cold spots. The result is outstanding temperature uniformity, which is critical for consistent material outcomes.
Fast and Precise Heating
The combination of high thermal conductivity and low thermal mass allows graphite crucible furnaces to heat up and cool down rapidly. Modern control systems leverage this responsiveness to manage temperatures with extreme precision, a non-negotiable requirement for sensitive material synthesis.
Key Advanced Material Applications
The physical properties of graphite directly enable its use in several cutting-edge fields. Each application leverages the furnace's ability to create a tightly controlled thermal environment.
Synthesis of Nanomaterials
The production of materials like graphene and carbon nanotubes often requires a process called Chemical Vapor Deposition (CVD). This process demands a stable, high-temperature environment to decompose precursor gases and grow highly ordered crystalline structures. The uniformity of a graphite furnace ensures consistent growth across the substrate.
Sintering of Advanced Ceramics and Powders
Sintering is a process where powdered material is heated to just below its melting point, causing the particles to fuse together into a solid, dense object. This is essential for creating high-strength ceramics and metal components. Temperature uniformity is paramount; any variation can lead to uneven density, internal stresses, and a failed part.
Graphitization and Heat Treatment
Graphitization is the process of converting amorphous carbon materials into crystalline graphite through sustained, high-temperature heat treatment. This is used to produce high-purity graphite electrodes and other components. The furnace provides the necessary stable, high-heat environment for this structural transformation to occur.
Precision Melting of Specialty Alloys
Industries like electronics and automotive rely on alloys with very specific compositions. Graphite crucibles are used to melt these materials because they heat quickly, allow for precise temperature control to prevent the loss of volatile alloy elements, and can be used in inert atmospheres to minimize oxidation.
Understanding the Trade-offs and Limitations
While powerful, graphite crucible furnaces are not a universal solution. An objective assessment requires acknowledging their operational constraints.
Oxidation in Atmosphere
Graphite will rapidly oxidize (burn away) in the presence of oxygen at high temperatures. For this reason, these furnaces must almost always be operated within a vacuum or an inert gas atmosphere (like argon or nitrogen), which adds complexity and cost to the system.
Reactivity with Certain Metals
Graphite is carbon. It can and will react with certain molten metals, notably iron and steel, introducing carbon into the melt. While this can be used intentionally for carburization, it is a source of contamination if pure, carbon-free metals are required.
Mechanical Brittleness
At room temperature, graphite is a brittle material. Crucibles can be susceptible to cracking from mechanical shock (being dropped) or severe thermal shock (extremely rapid, uneven heating or cooling). Careful handling and programmed heating cycles are essential.
Making the Right Choice for Your Goal
Selecting the right technology depends entirely on the specific properties your final material requires.
- If your primary focus is synthesizing novel materials: The unparalleled temperature uniformity and stability of a graphite furnace are essential for achieving reproducible, high-quality results in research and development.
- If your primary focus is manufacturing high-purity components: The fast heating and ability to operate in a controlled atmosphere make it ideal for producing specialty alloys and sintered parts where contamination is a critical concern.
- If your primary focus is general metal casting: For common non-ferrous metals like aluminum or bronze, the cost-effectiveness and operational simplicity of a basic crucible furnace may be sufficient without needing the advanced capabilities of graphite.
Ultimately, a graphite crucible furnace is a precision instrument designed for processes where temperature control is synonymous with material quality.
Summary Table:
| Application | Key Benefits |
|---|---|
| Nanomaterial Synthesis (e.g., graphene, carbon nanotubes) | Precise temperature control for uniform growth and reproducibility |
| Sintering of Advanced Ceramics and Powders | High temperature uniformity prevents defects and ensures dense parts |
| Graphitization and Heat Treatment | Stable high-heat environment for structural transformations |
| Precision Melting of Specialty Alloys | Fast heating and inert atmosphere to maintain alloy purity |
Ready to elevate your material processing with precision and reliability? At KINTEK, we leverage exceptional R&D and in-house manufacturing to provide advanced high-temperature furnace solutions tailored to your needs. Our product line—including Muffle, Tube, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems—is designed for labs focused on nanomaterials, ceramics, and specialty alloys. With strong deep customization capabilities, we ensure our furnaces meet your unique experimental requirements. Contact us today to discuss how KINTEK can enhance your research and production outcomes!
Visual Guide
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- Multi Zone Laboratory Quartz Tube Furnace Tubular Furnace
- Split Multi Heating Zone Rotary Tube Furnace Rotating Tube Furnace
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
People Also Ask
- Why is a tube furnace utilized for the heat treatment of S/C composite cathode materials? Optimize Battery Stability
- How is a high-temperature tube furnace utilized in the synthesis of MoO2/MWCNTs nanocomposites? Precision Guide
- What is an example of a material prepared using a tube furnace? Master Precise Material Synthesis
- What safety measures are essential when operating a lab tube furnace? A Guide to Preventing Accidents
- What recent improvements have been made to lab tube furnaces? Unlock Precision, Automation & Safety



















