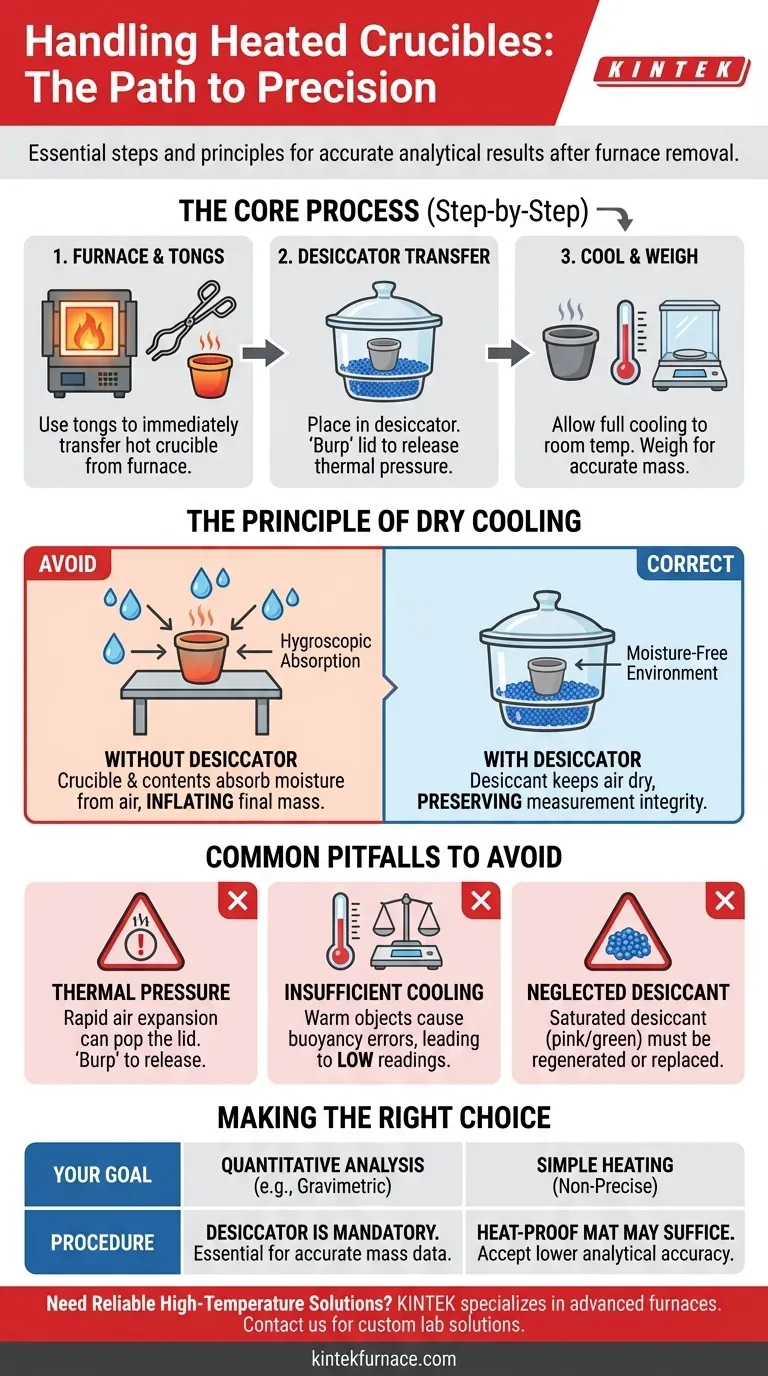
Upon removal from a furnace, a heated crucible must be handled with specific care to preserve the integrity of your analytical results. Using crucible tongs, you should immediately and carefully transfer the hot crucible to a desiccator to cool in a controlled, moisture-free environment before weighing.
The core reason for this procedure is to prevent the crucible and its contents from absorbing moisture from the atmosphere as they cool. This step is fundamental for ensuring that your final mass measurement is accurate and not artificially inflated by water.

The Principle of Dry Cooling
Handling a crucible after heating is a critical step in procedures like gravimetric analysis, where precision is paramount. The entire process is designed to eliminate one specific variable: atmospheric water vapor.
What is a Desiccator?
A desiccator is a sealable enclosure that contains a drying agent, or desiccant, such as silica gel or anhydrous calcium chloride. Its sole function is to maintain an atmosphere with very low humidity.
When a hot crucible is placed inside and the desiccator is sealed, the air trapped within is kept dry by the desiccant.
Why a Dry Environment is Critical
Many materials, including the porous ceramic of a crucible and the chemical residue being analyzed, are hygroscopic. This means they readily attract and absorb water molecules from the air.
This absorption happens most actively as an object cools from a high temperature down to room temperature. Leaving a crucible to cool on an open lab bench guarantees it will gain mass from water vapor, invalidating any precise measurement.
The Impact on Mass Measurement
In quantitative analysis, you are often measuring a mass difference to determine the amount of a substance. Even a minuscule amount of absorbed water—far too small to see—can significantly alter your results.
Failing to use a desiccator introduces a systemic error, leading to consistently high and inaccurate mass readings. This compromises the reliability and validity of the entire experiment.
Common Pitfalls to Avoid
Correctly using a desiccator involves more than just placing the crucible inside. Avoiding common mistakes is key to achieving accurate and repeatable results.
Thermal Pressure Changes
Placing a very hot crucible into a sealed desiccator will rapidly heat the air inside, causing it to expand. This pressure can cause the lid to pop off or prevent a proper seal from forming.
To prevent this, place the crucible inside and slide the lid almost closed, leaving a tiny gap. After a minute, "burp" the desiccator by sliding the lid open and closed again to release the pressure before sealing it completely.
Insufficient Cooling Time
A crucible must be cooled completely to room temperature before weighing. A warm object heats the air around it on the analytical balance pan, creating convective air currents.
These currents exert an upward force on the pan, making the object appear lighter than it actually is. This phenomenon, known as "buoyancy error," will lead to an inaccurate, low mass reading.
Neglecting Desiccant Maintenance
The desiccant is the active component of the system. Over time, it will become saturated with water and lose its effectiveness.
Many modern desiccants, like silica gel, contain a color indicator (typically changing from blue to pink or orange to green) that shows when it is saturated. Saturated desiccant must be regenerated by heating it in an oven or replaced to ensure the desiccator functions properly.
Making the Right Choice for Your Goal
The required procedure depends entirely on the objective of your work.
- If your primary focus is quantitative analysis (e.g., gravimetric analysis): Using a desiccator is non-negotiable for preventing moisture absorption and achieving accurate mass data.
- If your primary focus is simply heating a substance without precise final massing: While a desiccator is still best practice for material stability, cooling on a heat-proof mat in an area free from drafts may suffice, but you must accept that the final mass will not be analytically accurate.
Following this procedure transforms the simple act of cooling into a cornerstone of precise and repeatable scientific measurement.
Summary Table:
| Handling Step | Purpose | Key Considerations |
|---|---|---|
| Use Crucible Tongs | Safe transfer from furnace | Prevents burns and contamination |
| Place in Desiccator | Controlled, dry cooling | Avoids moisture absorption for accurate weighing |
| Allow Full Cooling | Reach room temperature | Prevents buoyancy errors in mass measurement |
| Maintain Desiccant | Ensure low humidity | Check for saturation and regenerate or replace as needed |
Need reliable high-temperature solutions for your lab? At KINTEK, we specialize in advanced furnaces like Muffle, Tube, Rotary, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems. Our strong R&D and in-house manufacturing allow for deep customization to meet your unique experimental needs. Ensure precise handling and accurate results—contact us today to discuss how our solutions can enhance your laboratory's efficiency and reliability!
Visual Guide
Related Products
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
People Also Ask
- What is nitrogen used for in a furnace? Prevent Oxidation and Control Heat Treatment Quality
- What is the main purpose of heat treatment? Transform Metal Properties for Superior Performance
- How does the inert atmosphere heat treating process work? Prevent Oxidation for Superior Material Quality
- How does a chemically inert atmosphere function in a furnace? Prevent Oxidation and Ensure Material Purity
- What does inert mean in furnace atmospheres? Protect materials from oxidation with inert gases.



















