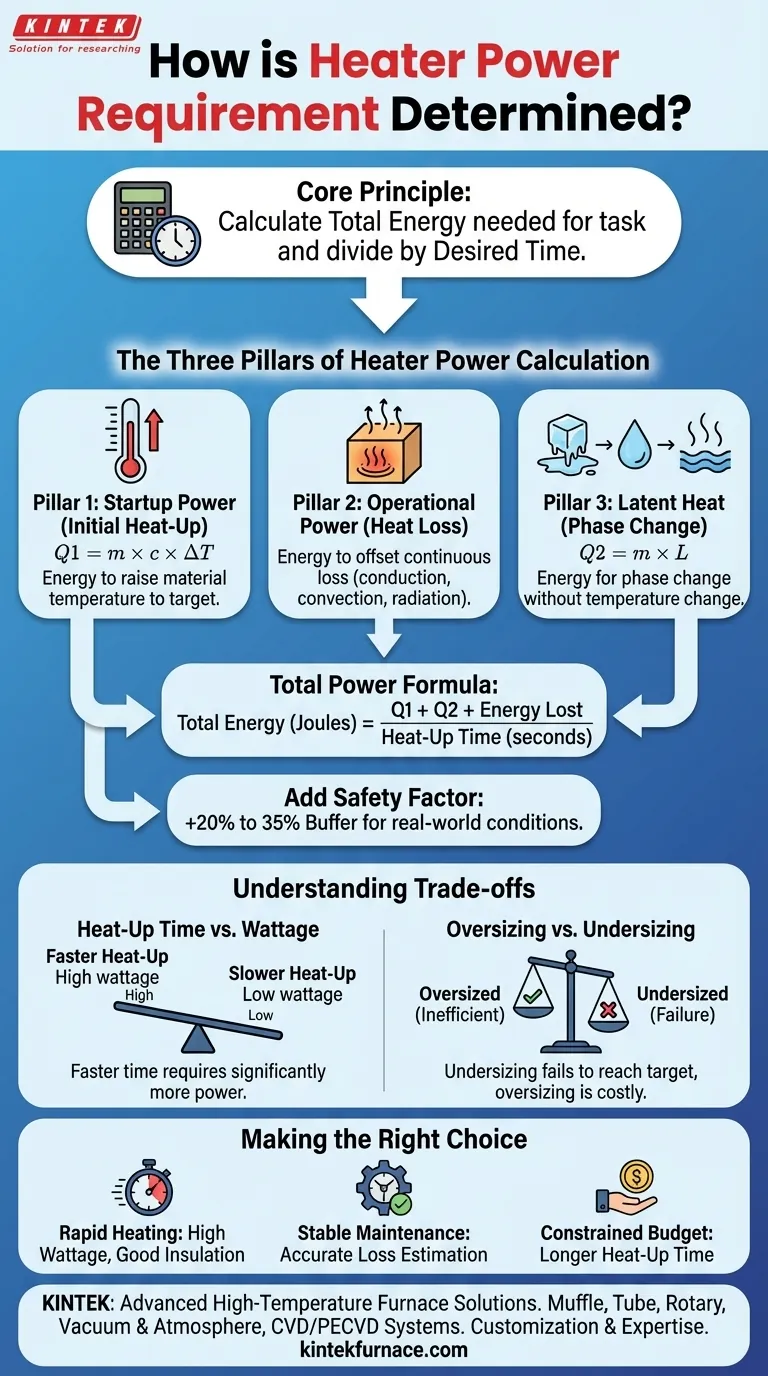
At its core, determining the power requirement for a heater involves calculating the total energy needed to perform a task and then dividing that energy by the desired time to complete it. This calculation must account for the initial energy to heat the substance, any energy required for phase changes (like melting or boiling), and the continuous energy needed to offset heat lost to the environment.
The crucial insight is that selecting a heater isn't just about reaching a target temperature. It's about designing an energy system that can overcome initial inertia (startup) and then precisely balance ongoing heat losses (maintenance) within your required timeframe.

The Three Pillars of Heater Power Calculation
To accurately determine the required power, you must calculate the energy needed for three distinct physical processes. The sum of this energy, when factored against your desired heat-up time, gives you your necessary power in watts.
Pillar 1: Startup Power (Initial Heat-Up)
This is the energy required to raise the temperature of the material from its starting point to the final target temperature.
It is calculated using the formula for sensible heat: Q1 = m × c × ΔT
- m: The mass of the material you are heating (e.g., in kilograms).
- c: The specific heat of the material, which is its capacity to store heat. This value is unique for every substance (water, steel, oil, etc.).
- ΔT: The change in temperature required (final temperature - initial temperature).
Pillar 2: Operational Power (Heat Loss)
Once the target temperature is reached, the heater’s job is to replace the heat that is constantly escaping to the cooler surroundings.
This ongoing heat loss occurs through conduction, convection, and radiation. Accurately calculating this is complex, but it is a critical factor for maintaining a stable temperature. This energy requirement is often expressed as a rate (e.g., Watts or BTU/hr).
Pillar 3: Latent Heat (Phase Change)
If the process involves a phase change, like melting a solid or boiling a liquid, you must account for the latent heat.
This is a significant amount of energy required to change the state of the material without changing its temperature. It is calculated as: Q2 = m × L
- m: The mass of the material changing phase.
- L: The latent heat of fusion (for melting) or vaporization (for boiling) for that specific substance.
Putting It All Together: The Total Power Formula
With the individual energy requirements understood, you can calculate the total power needed.
Step 1: Calculate Total Energy
First, sum the energy required for the initial startup and any phase changes. You also need to estimate the total heat that will be lost during the heat-up period.
Total Energy (Joules) = Q1 (Startup) + Q2 (Phase Change) + Energy Lost During Startup
Step 2: Convert Energy to Power
Power is simply energy divided by time. To find the required power in watts, divide the total energy (in Joules) by your desired heat-up time (in seconds).
Power (Watts) = Total Energy / Heat-Up Time (seconds)
This gives you the raw power needed to meet the demand without any buffer.
Step 3: Add a Safety Factor
You should never specify a heater for the exact calculated wattage. Real-world conditions like voltage fluctuations and unaccounted-for heat losses require a buffer.
A standard industry practice is to add a safety factor of 20% to 35% to the final calculated power. This ensures the heater can perform reliably under non-ideal conditions.
Understanding the Trade-offs
Choosing a heater involves balancing competing priorities. Understanding these trade-offs is key to making an objective decision.
Heat-Up Time vs. Wattage
The relationship is simple: a faster heat-up time demands significantly more power. Cutting the heat-up time in half can nearly double the required wattage, increasing both the heater's cost and the electrical infrastructure needed to support it.
Oversizing vs. Undersizing
Undersizing is a critical failure. An undersized heater may never reach the target temperature or will take an unacceptably long time to do so, especially in cold environments.
Oversizing is less critical but is inefficient. It leads to higher initial costs and can cause the temperature to overshoot the target, requiring more sophisticated controls to prevent temperature swings.
The Challenge of Accurate Loss Calculation
Calculating the initial startup and latent heat energies is straightforward. The most difficult and error-prone part of the process is accurately estimating the continuous heat loss during operation, as it depends heavily on insulation, ambient temperature, and air movement.
Making the Right Choice for Your Application
Use your primary goal to guide your final decision.
- If your primary focus is rapid heating: Invest in a high-wattage heater but also prioritize excellent insulation to minimize the power needed for temperature maintenance later.
- If your primary focus is stable temperature maintenance: Direct your efforts toward accurately estimating operational heat loss and select a heater that comfortably exceeds that value.
- If your primary focus is a constrained budget: Be prepared to accept a longer heat-up time, as this will directly reduce the required wattage and the initial cost of the heater.
By systematically accounting for every energy demand in your system, you can specify a heater that is both effective and efficient for your precise goal.
Summary Table:
| Component | Description | Formula |
|---|---|---|
| Startup Power | Energy to heat material to target temperature | Q1 = m × c × ΔT |
| Operational Power | Energy to offset continuous heat loss | Estimated based on insulation and environment |
| Latent Heat | Energy for phase changes (e.g., melting, boiling) | Q2 = m × L |
| Total Power | Sum of energies divided by heat-up time | Power = Total Energy / Time |
| Safety Factor | Buffer for real-world conditions | Add 20-35% to calculated power |
Struggling with heater power calculations for your lab? KINTEK leverages exceptional R&D and in-house manufacturing to provide advanced high-temperature furnace solutions, including Muffle, Tube, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems. With strong deep customization capabilities, we precisely meet your unique experimental requirements. Contact us today to optimize your heating processes and enhance efficiency!
Visual Guide
Related Products
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- Multi Zone Laboratory Quartz Tube Furnace Tubular Furnace
People Also Ask
- What is the primary use of a muffle furnace in the assembly of side-heated resistive gas sensors? Expert Annealing Guide
- What role does a muffle furnace play in the conversion of S-1@TiO2? Achieve Precision Calcination of Nanospheres
- What role does a muffle furnace play in g-C3N4 synthesis? Mastering Thermal Polycondensation for Semiconductors
- Why is a muffle furnace used to determine the ash content of biochar? Master Your Material Purity Analysis
- How does a stainless steel reactor function within a muffle furnace for PET to graphene? Master Carbon Synthesis



















