In a chemistry lab, a box muffle furnace is a powerful tool for high-temperature transformations. It is most commonly used for specific chemical reactions like synthesis, pyrolysis (thermal decomposition), and calcination, as well as for analytical techniques such as ashing. These processes all rely on the furnace's ability to achieve and maintain extreme temperatures in a highly controlled manner.
The true value of a muffle furnace is not just its heat, but its ability to provide a thermally isolated and contaminant-free environment. This allows chemists to transform materials at extreme temperatures without interference from the heating source itself, ensuring the purity and integrity of the experiment.

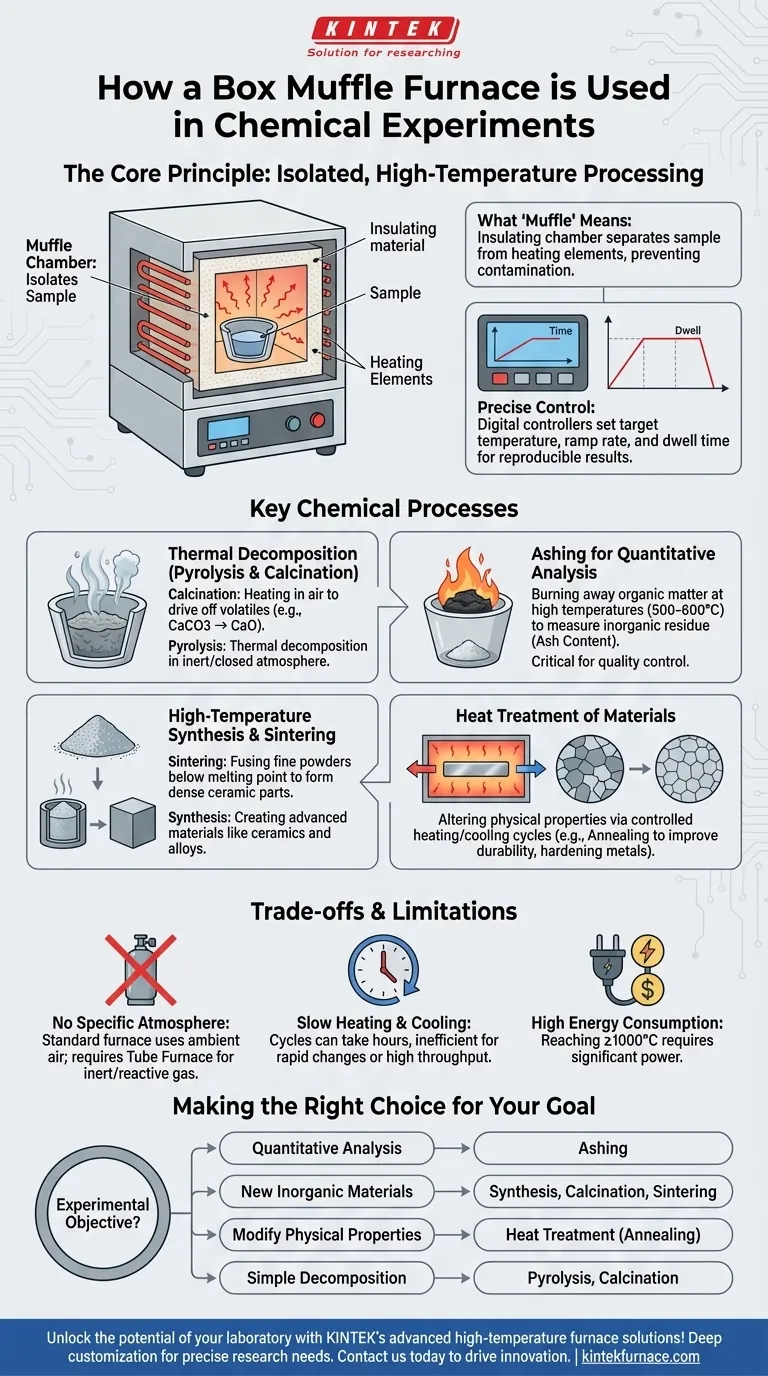
The Core Principle: Isolated, High-Temperature Processing
A muffle furnace is fundamentally different from a standard oven. Its design is centered on providing pure, radiant heat to a sample.
What "Muffle" Means
The term muffle refers to the insulating chamber, typically made of high-purity ceramic, that separates the sample from the heating elements.
This design is critical because it prevents any direct contact or contamination. The heating elements heat the chamber, and the chamber, in turn, radiates that heat evenly onto the sample inside.
The Importance of Precise Control
Modern box muffle furnaces are equipped with digital controllers that allow for precise temperature programming.
Chemists can set specific target temperatures, control the rate of heating (ramp rate), and maintain a temperature for a set duration (dwell time). This level of control is essential for reproducible results in material synthesis and analysis.
Key Chemical Processes in a Muffle Furnace
The furnace's unique environment enables several core laboratory procedures that are difficult or impossible to perform with other heating equipment.
Thermal Decomposition (Pyrolysis and Calcination)
Many experiments require breaking down compounds using heat. A muffle furnace is ideal for this.
Calcination is the process of heating a solid to high temperatures in air to induce a chemical change, such as driving off water or carbon dioxide. A classic example is heating calcium carbonate to produce calcium oxide.
Pyrolysis is similar but refers to the thermal decomposition of materials at elevated temperatures in an inert atmosphere (though often performed in the limited air of a closed furnace).
Ashing for Quantitative Analysis
Ashing is one of the most common applications in analytical chemistry. The process involves completely burning away all organic matter from a sample at high temperatures (e.g., 500-600°C).
The small amount of inorganic residue left behind is the ash content. This is a critical quality control metric in the pharmaceutical, environmental, and food industries.
High-Temperature Synthesis and Sintering
Creating advanced materials like ceramics, alloys, or specialized glass often requires extreme heat.
Sintering is a key process where fine powders are heated to just below their melting point. The heat causes the particles to fuse together, forming a solid, dense object. This is fundamental to manufacturing most ceramic parts.
Heat Treatment of Materials
The furnace is also used to alter the physical properties of a material without changing its chemical composition.
Processes like annealing or hardening metals involve carefully controlled heating and cooling cycles. These treatments modify the material's internal crystal structure, changing its durability, flexibility, or strength.
Understanding the Trade-offs and Limitations
While powerful, a muffle furnace is not the right tool for every job. Understanding its limitations is key to using it effectively.
Not for Reactions Requiring a Specific Atmosphere
A standard box muffle furnace heats samples in the ambient air inside its chamber. It cannot, by default, be used for reactions that require an inert (e.g., argon) or reactive (e.g., hydrogen) gas atmosphere. For those applications, a specialized tube furnace is the correct instrument.
Slow Heating and Cooling Cycles
The very insulation that makes a muffle furnace so stable also means it heats up and cools down very slowly. A cycle can take several hours. This makes it inefficient for high-throughput work or experiments that require rapid temperature changes.
Significant Energy Consumption
Reaching and maintaining temperatures of 1000°C or higher requires a tremendous amount of electrical energy. These are power-intensive devices that can have a notable impact on a lab's operational costs.
Making the Right Choice for Your Goal
To use a muffle furnace correctly, match its capabilities to your experimental objective.
- If your primary focus is quantitative analysis: Use the furnace for ashing to determine the inorganic content of a sample for quality control.
- If your primary focus is creating new inorganic materials: Use the furnace for high-temperature synthesis, calcination to create oxides, or sintering to form dense ceramic parts.
- If your primary focus is modifying a material's physical properties: Use the furnace for heat treatment processes like annealing to improve a material's structural characteristics.
- If your primary focus is simple thermal decomposition: Use the furnace for pyrolysis or calcination to break down compounds in a controlled, high-heat environment.
By understanding these core functions, you can leverage the muffle furnace as a precise and powerful tool for material transformation and analysis.
Summary Table:
| Process | Description | Common Applications |
|---|---|---|
| Pyrolysis | Thermal decomposition in inert atmosphere | Breaking down compounds |
| Calcination | Heating in air to drive off volatiles | Producing oxides from carbonates |
| Ashing | Burning organic matter to measure inorganic residue | Quality control in food, pharma, and environmental analysis |
| Sintering | Fusing powders below melting point | Manufacturing ceramics and dense materials |
| Heat Treatment | Altering physical properties via heating cycles | Annealing metals for improved durability |
Unlock the full potential of your laboratory with KINTEK's advanced high-temperature furnace solutions! Leveraging exceptional R&D and in-house manufacturing, we provide diverse labs with reliable equipment like Muffle, Tube, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems. Our strong deep customization capability ensures precise solutions for your unique experimental needs, enhancing efficiency and accuracy in processes such as pyrolysis, calcination, and sintering. Contact us today to discuss how we can support your research and drive innovation in your lab!
Visual Guide
Related Products
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- Multi Zone Laboratory Quartz Tube Furnace Tubular Furnace
People Also Ask
- Why is a high-performance muffle furnace required for the calcination of nanopowders? Achieve Pure Nanocrystals
- What is the primary function of a muffle furnace for BaTiO3? Master High-Temp Calcination for Ceramic Synthesis
- What metals cannot be heated by induction? Understanding Material Suitability for Efficient Heating
- What role does a muffle furnace play in the preparation of MgO support materials? Master Catalyst Activation
- How does a laboratory muffle furnace facilitate the biomass carbonization process? Achieve Precise Biochar Production



















