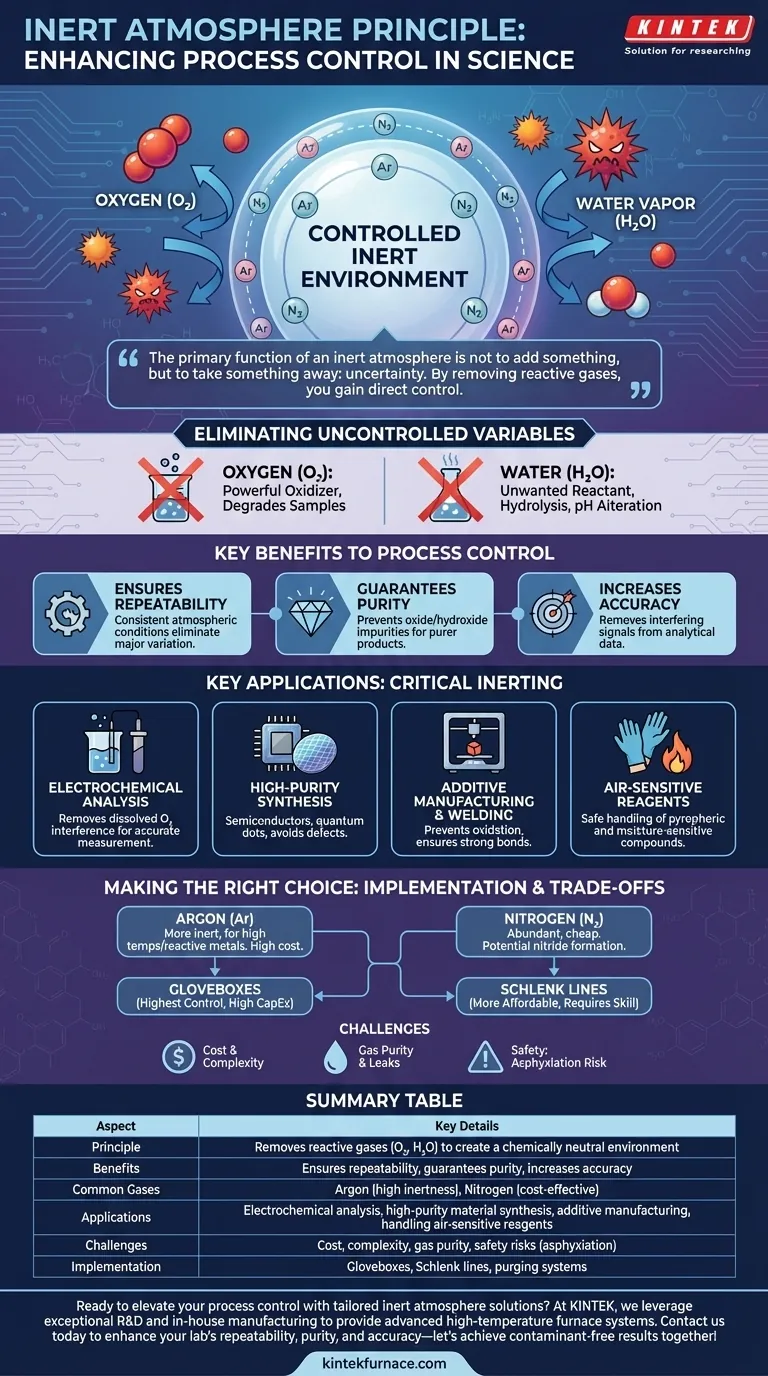
At its core, the inert atmosphere principle enhances process control by creating a chemically neutral background. This controlled environment systematically removes reactive atmospheric gases, primarily oxygen and water vapor, that would otherwise introduce unwanted and unpredictable side reactions. By eliminating these variables, you ensure that the process you observe is the one you intended to run.
The primary function of an inert atmosphere is not to add something to your process, but to take something away: uncertainty. By removing reactive atmospheric gases, you gain direct control over the chemical environment, ensuring that your results are a true reflection of your intended experiment, not a product of contamination.

The Principle of Inerting: Eliminating Uncontrolled Variables
To master process control, you must first define the boundaries of your system. An inert atmosphere is one of the most powerful tools for establishing a stable, predictable chemical boundary.
What Is an Inert Atmosphere?
An inert atmosphere is a volume of gas that does not readily participate in chemical reactions under a given set of conditions. This environment is typically composed of noble gases like Argon (Ar) or a relatively non-reactive gas like Nitrogen (N₂).
The goal is to physically displace the reactive air in a workspace—such as a glovebox, reactor, or Schlenk line—with this non-reactive gas, creating a controlled experimental stage.
The Primary Contaminants: Oxygen and Water
For most applications, the two main culprits you are trying to eliminate from ambient air are oxygen and water vapor.
Oxygen (O₂) is a powerful oxidizing agent that can degrade samples, form unwanted oxides, or interfere with electrochemical measurements.
Water (H₂O) can act as an unwanted reactant in hydrolysis reactions, a catalyst for side reactions, or a source of protons that can alter a system's pH.
How This Translates to Process Control
By purging a system with an inert gas, you remove these uncontrolled variables. This directly enhances process control in three fundamental ways:
- Ensures Repeatability: Every experiment is run under the exact same atmospheric conditions, eliminating a major source of variation.
- Guarantees Purity: It prevents the formation of oxide or hydroxide impurities in synthesized materials, leading to a purer final product.
- Increases Accuracy: It removes interfering signals from analytical measurements, ensuring the data you collect is a true representation of your sample.
Key Applications Where Inerting is Critical
The need for a controlled atmosphere is not academic; it is a practical necessity in many high-stakes scientific and industrial fields.
Electrochemical Analysis
As the reference notes, this is a classic example. Oxygen dissolved in an electrolyte is electrochemically active and can create a background signal that masks the signal of the analyte you are trying to measure. Purging the solution with Argon removes this interference, leading to a clean, accurate measurement.
High-Purity Material Synthesis
In fields like semiconductor manufacturing or quantum dot synthesis, even parts-per-million levels of oxygen can introduce defects into a crystal lattice. These defects can drastically alter the material's electronic or optical properties, rendering it useless.
Additive Manufacturing and Welding
When working with metals at high temperatures, such as in 3D metal printing or TIG welding, exposure to oxygen causes rapid oxidation. This forms a brittle, weak oxide layer that compromises the structural integrity of the final part. A continuous shield of inert gas is required to create strong, clean bonds.
Handling of Air-Sensitive Reagents
Many organometallic compounds and other advanced reagents are pyrophoric (ignite spontaneously in air) or rapidly decompose on exposure to moisture. For these chemicals, an inert atmosphere is not just for process control—it is an absolute requirement for safe handling and experimental viability.
Understanding the Trade-offs and Implementation Challenges
While powerful, implementing an inert atmosphere system is not without its challenges. A clear understanding of the trade-offs is essential for effective use.
Choosing the Right Inert Gas
Nitrogen is the workhorse of inerting because it is abundant and inexpensive. However, at high temperatures, it can react with certain metals (like lithium or titanium) to form nitrides.
Argon is significantly more inert than nitrogen and is the gas of choice for high-temperature processes or when working with highly reactive metals. Its primary drawback is its higher cost.
Cost and Complexity
Achieving and maintaining an inert atmosphere requires specialized equipment. Gloveboxes offer the highest level of control but are a significant capital investment. Schlenk lines are a more affordable alternative for chemistry labs but require more user skill. The continuous consumption of high-purity gas also represents an ongoing operational cost.
Purity Is Not Absolute
Inert gases are sold in various purity grades. For highly sensitive applications, even "ultra-high purity" gas may need to pass through an additional gas purifier to remove trace contaminants down to parts-per-billion levels. Furthermore, maintaining this purity is a constant battle against microscopic leaks in the system.
Safety Considerations
Inert gases are asphyxiants. A major leak in a poorly ventilated room can displace oxygen to dangerously low levels. All facilities using large quantities of inert gas must have robust ventilation and be equipped with oxygen level monitors and alarms.
Making the Right Choice for Your Application
The stringency of your inerting strategy should be directly proportional to the sensitivity of your process.
- If your primary focus is analytical precision: Use the highest purity gas (typically Argon) within a sealed system like a glovebox to eliminate even trace electrochemical or spectroscopic interference.
- If your primary focus is bulk material integrity: Nitrogen is often the most cost-effective choice for processes like welding or annealing, but you must first verify it does not form unwanted nitrides with your material at process temperatures.
- If your primary focus is safety when handling reactive chemicals: A robust, leak-tested glovebox or Schlenk line is non-negotiable to prevent decomposition, fire, or explosions.
- If your primary focus is long-term sample stability: Storing sensitive materials under a positive pressure of an inert gas inside a desiccator or glovebox prevents degradation from slow oxidation or hydrolysis over time.
Ultimately, mastering the use of an inert atmosphere transforms it from a complex requirement into a powerful tool for achieving absolute process fidelity.
Summary Table:
| Aspect | Key Details |
|---|---|
| Principle | Removes reactive gases (O₂, H₂O) to create a chemically neutral environment |
| Benefits | Ensures repeatability, guarantees purity, increases accuracy |
| Common Gases | Argon (high inertness), Nitrogen (cost-effective) |
| Applications | Electrochemical analysis, high-purity material synthesis, additive manufacturing, handling air-sensitive reagents |
| Challenges | Cost, complexity, gas purity, safety risks (asphyxiation) |
| Implementation | Gloveboxes, Schlenk lines, purging systems |
Ready to elevate your process control with tailored inert atmosphere solutions? At KINTEK, we leverage exceptional R&D and in-house manufacturing to provide advanced high-temperature furnace systems, including Muffle, Tube, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems. Our deep customization capabilities ensure precise alignment with your unique experimental needs, whether you're in electrochemistry, material synthesis, or handling air-sensitive reagents. Contact us today to discuss how our expertise can enhance your lab's repeatability, purity, and accuracy—let's achieve contaminant-free results together!
Visual Guide
Related Products
- Cylindrical Resonator MPCVD Machine System for Lab Diamond Growth
- MPCVD Machine System Reactor Bell-jar Resonator for Lab and Diamond Growth
- 915MHz MPCVD Diamond Machine Microwave Plasma Chemical Vapor Deposition System Reactor
- RF PECVD System Radio Frequency Plasma Enhanced Chemical Vapor Deposition
- Multi Heating Zones CVD Tube Furnace Machine for Chemical Vapor Deposition Equipment
People Also Ask
- What are some challenges associated with MPCVD? Overcome High Costs and Complexity for Diamond Synthesis
- How is MPCVD used in the production of polycrystalline diamond optical components? Discover High-Purity Diamond Growth for Optics
- Why is keeping maintenance records important for MPCVD equipment? Ensure Reliability and Quality in Crystal Growth
- What is the role of inert gas doping in the MPCVD method? Accelerate Single-Crystal Diamond Growth
- What are some applications of MPCVD? Unlock High-Purity Diamond for Advanced Engineering



















