At its core, high-temperature stability is the single most critical property of a graphite heating element because it ensures the material can perform its primary function—generating intense heat—without degrading, melting, or failing. This stability allows for reliable and repeatable performance in extreme industrial environments, such as vacuum furnaces operating at temperatures that would destroy most metals.
High-temperature stability is not merely about surviving heat; it's about maintaining structural integrity and predictable electrical properties under thermal stress. This predictability, combined with graphite's other unique thermal and chemical characteristics, makes it a superior and versatile material for advanced heating applications.

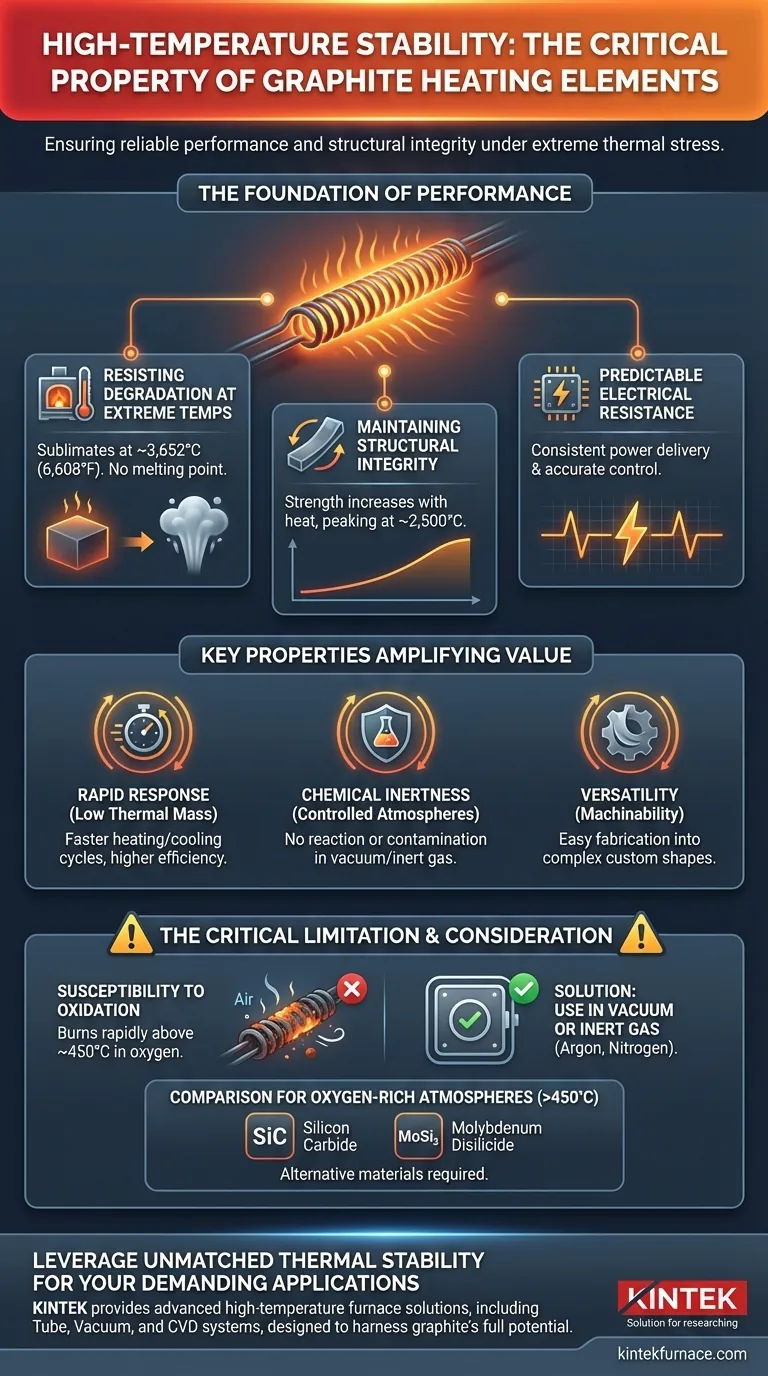
The Foundation of Performance: Unpacking High-Temperature Stability
The term "stability" encompasses several distinct properties that work together to make graphite an exceptional material for high-heat applications. Understanding these individual components reveals why it is so heavily relied upon in demanding industries.
Resisting Degradation at Extreme Temperatures
Graphite does not have a conventional melting point at atmospheric pressure; instead, it sublimates at approximately 3,652°C (6,608°F). This is significantly higher than the melting point of most industrial metals.
This exceptional thermal resilience makes it the material of choice for processes like metal sintering, hardening, and high-temperature brazing, where furnace temperatures must be both extreme and precisely controlled.
Maintaining Structural Integrity
Unlike many materials that weaken or deform as they heat up, graphite exhibits a unique and highly valuable trait: its mechanical strength increases with temperature, peaking around 2,500°C (4,532°F).
This means the heating element not only resists failure but becomes stronger and more rigid in its operating range, ensuring it maintains its shape and position within the furnace assembly.
Ensuring Predictable Electrical Resistance
A heating element works by converting electrical energy into heat through resistance. For precise temperature control, this resistance must be stable and predictable across the entire operating temperature range.
Graphite's stability ensures its electrical properties do not fluctuate erratically at high temperatures. This allows for consistent power delivery and highly accurate thermal cycling, which is critical for producing high-quality parts.
Key Properties That Amplify Graphite's Value
High-temperature stability is the cornerstone, but several other properties work in synergy to make graphite a uniquely effective and efficient heating material.
Low Thermal Mass for Rapid Response
Graphite has a relatively low thermal mass, meaning it requires less energy to heat up and it cools down quickly.
This enables rapid heating and cooling cycles, increasing furnace throughput and overall energy efficiency. It gives process engineers precise control over the thermal profile of a manufacturing run.
Chemical Inertness in Controlled Atmospheres
In the vacuum or inert gas environments common to high-temperature furnaces, graphite is chemically inert.
It will not react with or contaminate the products being processed, which is essential for applications in electronics, aerospace, and medical device manufacturing where material purity is paramount.
Versatility Through Machinability
High-purity, isostatic graphite is homogenous and consistent, making it remarkably easy to machine.
This allows heating elements, fixtures, and crucibles to be fabricated into complex and custom shapes, enabling optimized furnace designs and uniform heating patterns that would be difficult or impossible with other materials.
Understanding the Trade-offs and Considerations
While powerful, graphite is not a universal solution. Acknowledging its limitations is key to using it effectively and safely.
The Critical Role of Atmosphere
The single greatest limitation of graphite is its susceptibility to oxidation. In the presence of oxygen, graphite will begin to rapidly burn away at temperatures above approximately 450°C (842°F).
For this reason, graphite heating elements are used almost exclusively in vacuum furnaces or furnaces filled with an inert gas like argon or nitrogen. This protects the element from premature failure.
Comparison to Other High-Temperature Materials
For applications requiring high heat in an oxidizing atmosphere, engineers must turn to more exotic and often more expensive materials.
Materials like molybdenum disilicide (MoSi₂) or silicon carbide (SiC) can operate at very high temperatures in the open air, a task for which graphite is fundamentally unsuited.
The Importance of Material Quality
The performance of a graphite element is directly tied to the quality of the raw material and the precision of the machining process.
Using a low-purity grade or poorly machined element can lead to hotspots, inconsistent heating, and premature failure. Skilled machining and high-quality isostatic graphite are essential for reliability.
Making the Right Choice for Your Process
Selecting the correct heating element material requires a clear understanding of your process requirements and operating environment.
- If your primary focus is high-temperature processing in a vacuum or inert atmosphere: Graphite is an excellent choice due to its unmatched thermal stability, rapid response, and cost-effectiveness.
- If your process involves an oxygen-rich atmosphere above 450°C: You must consider alternative materials like silicon carbide or molybdenum disilicide, as graphite will rapidly oxidize and fail.
- If you require complex element shapes and precise temperature control: Graphite's superior machinability and stable electrical properties make it a highly adaptable and reliable solution.
Ultimately, understanding graphite's unique combination of properties empowers you to leverage its strengths for efficient and reliable high-temperature industrial processes.
Summary Table:
| Property | Benefit for Graphite Heating Elements |
|---|---|
| High Sublimation Point (~3650°C) | Resists degradation at extreme temperatures, ideal for sintering and brazing. |
| Increased Strength with Heat | Maintains structural integrity and shape, peaking around 2500°C. |
| Stable Electrical Resistance | Enables precise temperature control and consistent thermal cycling. |
| Low Thermal Mass | Allows for rapid heating/cooling cycles, improving energy efficiency. |
| Chemical Inertness | Prevents contamination of sensitive materials in vacuum/inert atmospheres. |
Leverage Unmatched Thermal Stability for Your Demanding Applications
Graphite's superior high-temperature performance is foundational for processes requiring extreme heat and precision. At KINTEK, we leverage our exceptional R&D and in-house manufacturing to provide advanced, high-temperature furnace solutions tailored to your unique needs.
Our product line, including high-performance Tube Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems, is designed to harness the full potential of graphite heating elements. Combined with our strong deep customization capability, we ensure your furnace system precisely meets your experimental and production requirements.
Ready to achieve reliable, high-temperature processing? Contact our experts today to discuss how our solutions can enhance your lab's efficiency and results.
Visual Guide
Related Products
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
- Silicon Carbide SiC Thermal Heating Elements for Electric Furnace
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
People Also Ask
- What is the significance of vacuum in relation to graphite components in furnaces? Prevent Oxidation for Extreme Temperatures
- What is the primary application of vacuum heat treating furnaces in aerospace? Enhance Component Performance with Precision
- Why are vacuum furnaces used for the re-quenching of samples after a boriding treatment? Master Core Toughness
- What is the mechanism and effect of post-annealing NiTi thin films in a vacuum furnace? Unlock Superelasticity
- How does vacuum heat treatment reduce workpiece deformation? Achieve Superior Dimensional Stability













