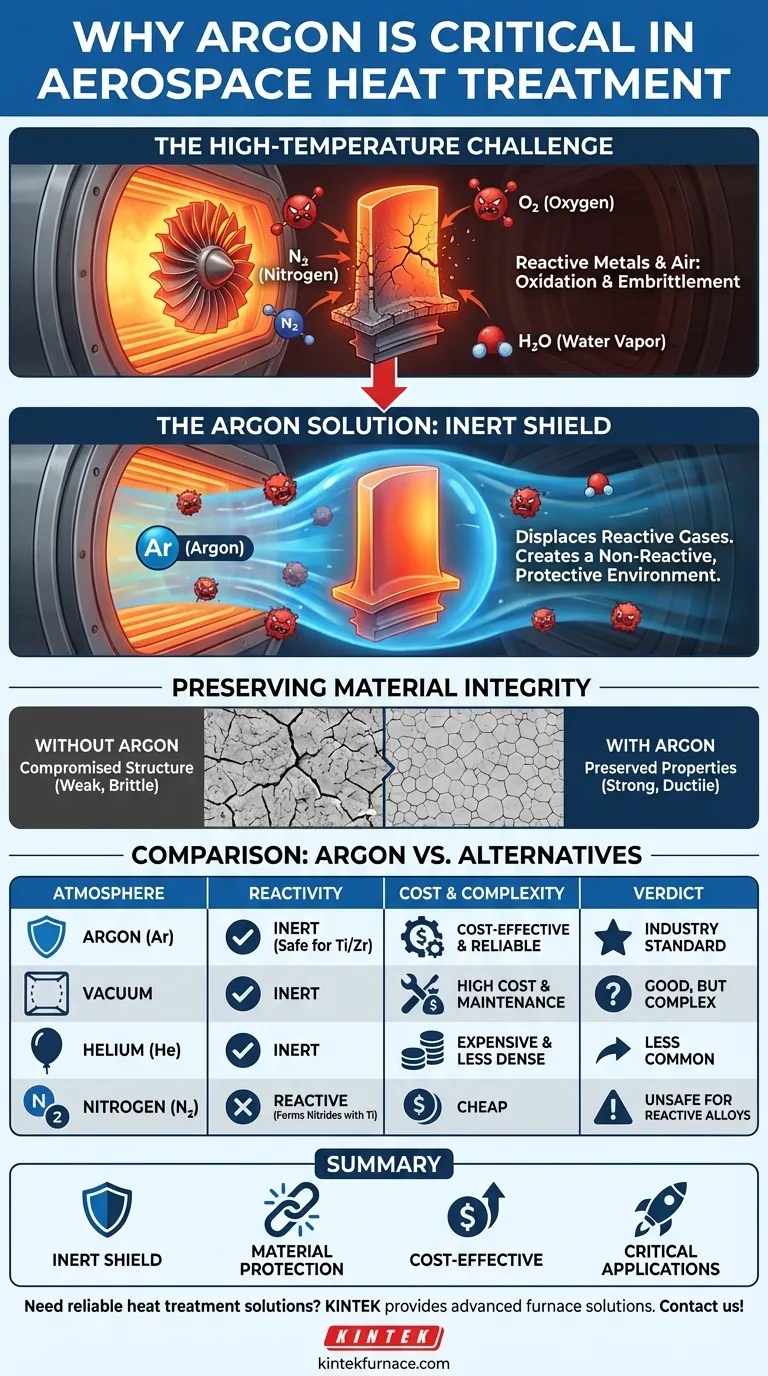
In the high-stakes world of aerospace engineering, argon is critical for heat treatment because it provides a completely inert atmosphere. High-performance alloys used in aerospace, such as titanium and zirconium, are highly reactive at elevated temperatures. Argon gas shields these metals from oxygen, nitrogen, and water vapor, preventing chemical reactions that would otherwise weaken the material and compromise the safety and performance of the final component.
The core challenge in aerospace manufacturing is not just shaping metal, but preserving its designed properties during high-temperature processing. Argon is the industry's solution because it provides an effective, reliable, and cost-efficient shield against atmospheric contamination, ensuring a component's structural integrity is never in question.

The Fundamental Challenge: Reactive Metals at High Temperatures
Heat treatment is a foundational process in metallurgy, used to alter a material's properties like hardness, strength, and ductility. However, the very heat that makes this possible also creates a significant risk.
The Problem with Air
The air we breathe is a mixture of gases, primarily nitrogen and oxygen, with traces of water vapor and other elements. At room temperature, these gases are relatively harmless to most metals.
At the extreme temperatures required for heat treatment, however, these gases become highly reactive. Oxygen, in particular, aggressively seeks to bond with metal atoms in a process called oxidation.
The Consequence of Contamination
For mission-critical aerospace alloys like titanium, this reactivity is a catastrophic liability. When heated in the presence of air, these metals can form a brittle, oxygen-rich surface layer.
This contamination leads to a loss of ductility and a severe reduction in fatigue life, a phenomenon known as embrittlement. A component that has been compromised in this way is unfit for aerospace use, as it could fail under operational stress.
How Argon Solves the Problem
The solution is to remove the reactive atmosphere entirely and replace it with a gas that will not interact with the metal, regardless of the temperature. This is where argon excels.
The Principle of Inertness
Argon is a noble gas, meaning its atomic structure makes it chemically inert. It does not react with other elements, even under the intense energy of a heat treatment furnace.
By flooding the furnace chamber with pure argon, manufacturers purge all the reactive atmospheric gases. This creates a protective, non-reactive environment surrounding the component.
Preserving Material Integrity
Within this argon shield, the heat treatment process can proceed as intended. The high temperatures can alter the metal's crystalline structure to achieve the desired strength and durability without any competing chemical reactions.
The result is a finished part that possesses the exact metallurgical properties specified by its designers, free from the defects and weaknesses caused by atmospheric contamination.
Understanding the Trade-offs: Argon vs. Other Atmospheres
While argon is a dominant choice, it's important to understand why it's often selected over other potential solutions like a vacuum or different inert gases.
Argon vs. A Vacuum
A high-vacuum furnace, which removes nearly all gas molecules, is another effective method for preventing contamination. However, vacuum systems are mechanically complex, require more maintenance, and can have higher capital and operational costs than argon-based systems. Argon offers a more flexible and often more economical solution.
Argon vs. Helium
Helium is also an inert noble gas. However, argon is about ten times more abundant in Earth's atmosphere, making it significantly more cost-effective and readily available. Furthermore, argon is denser than air, which can help it displace atmospheric gases more effectively in certain furnace designs, providing superior coverage.
Argon vs. Nitrogen
Nitrogen is relatively inert and much cheaper than argon. While suitable for many steels, it is not a safe choice for reactive alloys like titanium. At high temperatures, nitrogen can react with titanium to form titanium nitrides, which can also make the material brittle. Argon's complete inertness makes it the safer, more reliable choice for the most sensitive aerospace applications.
Making the Right Choice for Your Application
Selecting the correct protective atmosphere is a critical decision based on your material, performance requirements, and budget.
- If your primary focus is processing highly reactive alloys like titanium or zirconium: Argon is the non-negotiable standard for preventing embrittlement and ensuring maximum material performance.
- If your primary focus is cost-optimization for less reactive metals: You can evaluate nitrogen, but you must first confirm it will not form undesirable nitrides with your specific alloy at the target temperature.
- If your primary focus is achieving the absolute highest purity environment: A high-vacuum furnace is a valid alternative, though it often comes with higher capital and operational costs compared to an argon atmosphere.
Ultimately, selecting the correct protective atmosphere is a foundational step in guaranteeing the safety and reliability of critical aerospace systems.
Summary Table:
| Aspect | Role of Argon in Aerospace Heat Treatment |
|---|---|
| Inert Atmosphere | Provides a non-reactive shield against oxygen, nitrogen, and water vapor to prevent chemical reactions. |
| Material Protection | Safeguards reactive alloys like titanium and zirconium from oxidation and embrittlement at high temperatures. |
| Cost-Effectiveness | More abundant and economical than alternatives like helium or vacuum systems, ensuring reliable performance. |
| Application Suitability | Ideal for critical aerospace components where material integrity and safety are paramount. |
Need reliable heat treatment solutions for your aerospace projects? At KINTEK, we leverage exceptional R&D and in-house manufacturing to provide advanced high-temperature furnace solutions, including Muffle, Tube, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems. Our strong deep customization capability ensures we precisely meet your unique experimental requirements, helping you achieve superior material performance and safety. Contact us today to discuss how our expertise can support your critical applications!
Visual Guide
Related Products
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
People Also Ask
- How does the pressure range change under vacuum conditions in an atmosphere box furnace? Explore Key Shifts for Material Processing
- How does a mixed gas flow control system maintain stability during high-temperature nitriding? Precision Gas Ratios
- What are the development prospects of atmosphere box furnaces in the aerospace industry? Unlock Advanced Material Processing for Aerospace Innovation
- What is an atmosphere protection muffle furnace? Unlock Precise Heat Treatment in Controlled Environments
- What is inert gas technology used for in high-temperature atmosphere vacuum furnaces? Protect Materials and Speed Up Cooling



















