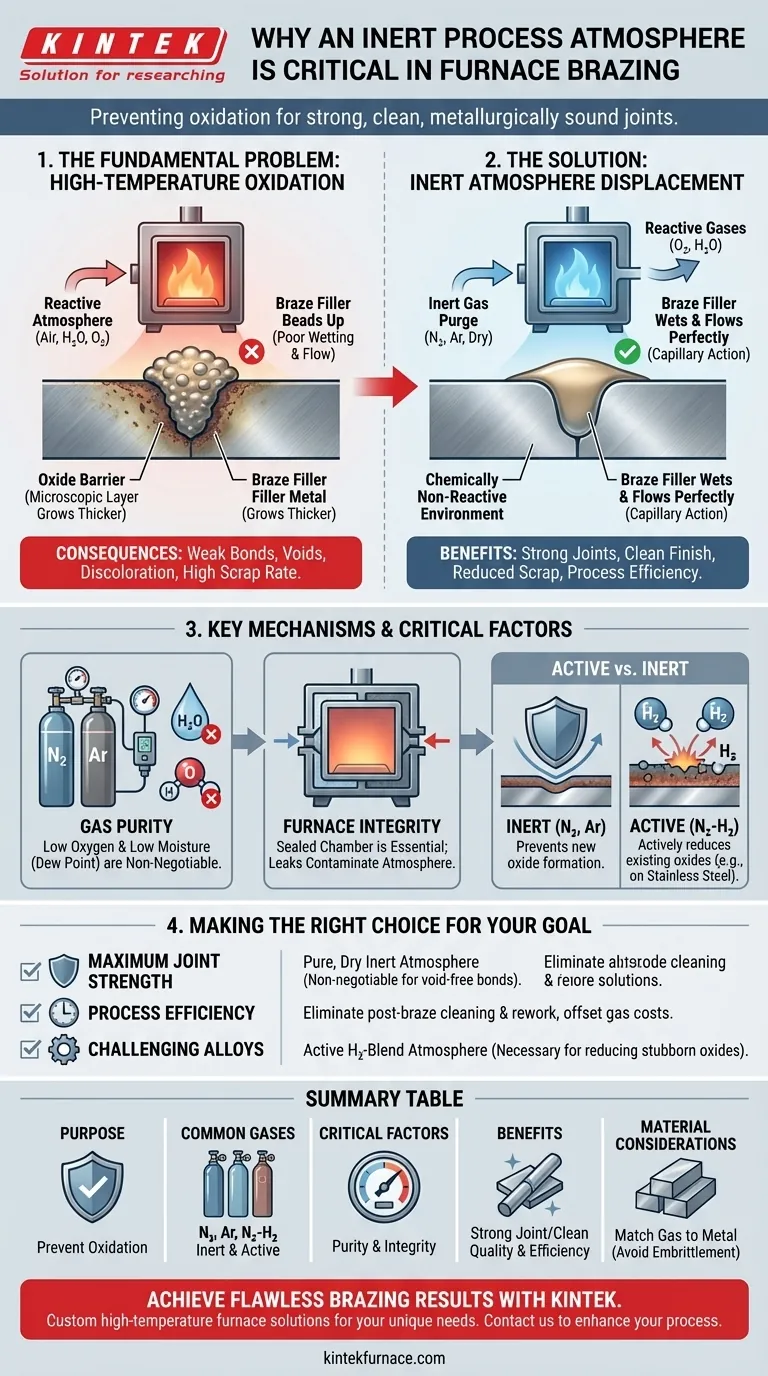
In furnace brazing, an inert atmosphere is essential for preventing the oxidation of metal surfaces at high temperatures. By displacing oxygen and moisture with a gas like dry nitrogen or argon, you ensure the braze filler metal can properly wet and flow, creating a strong, clean, and metallurgically sound joint. Without it, the process would fail.
The core purpose of an inert atmosphere is to create a chemically non-reactive environment during the heating cycle. This isn't just a best practice; it is the fundamental mechanism that allows for high-quality, repeatable brazing by eliminating the oxide layers that prevent a successful bond.

The Fundamental Problem: Oxidation at High Temperatures
What Happens to Metals When Heated?
All common engineering metals, with the exception of noble metals, have a natural affinity for oxygen. This reaction, known as oxidation, is dramatically accelerated by the high temperatures required for brazing.
Even a visually clean part is covered by a microscopic, transparent oxide layer. As you heat the part in the presence of air, this layer grows thicker and more tenacious.
The Role of the Oxide Barrier
This oxide layer acts as a physical barrier. The molten braze filler metal cannot wet or bond with the underlying parent metal; instead, it will bead up on the oxide surface, much like water on a waxed car.
This failure to wet and flow through the joint via capillary action is the primary cause of brazing defects, leading to weak or nonexistent bonds.
Consequences of Poor Atmosphere Control
Operating without a proper inert atmosphere results in predictable failures. You will see incomplete braze flow, voids within the joint, and significant discoloration.
These parts will either require extensive and costly post-braze cleaning or be scrapped entirely, leading to wasted time, material, and energy.
How an Inert Atmosphere Solves the Problem
Displacing Reactive Gases
The principle is simple: an inert atmosphere furnace works by first creating a sealed environment and then purging it with a non-reactive gas, most commonly nitrogen or argon.
This continuous flow of inert gas displaces the oxygen, moisture, and other reactive gases from the furnace chamber, protecting the parts throughout the entire heating and cooling cycle.
The Critical Importance of Gas Purity
A successful inert atmosphere depends on two factors: low oxygen content and low moisture content. The moisture level, often measured as dew point, is just as critical as the oxygen level.
Water vapor (H₂O) is a potent oxidizing agent at brazing temperatures. This is why using a dry inert gas is non-negotiable for achieving a clean, "bright" finish on the parts.
Active vs. Inert Atmospheres
In some cases, particularly with stainless steels that form stubborn chromium oxides, a purely inert gas isn't enough. An "active" atmosphere may be used.
These are typically nitrogen-hydrogen (N₂-H₂) blends. The hydrogen actively reacts with and reduces surface oxides, cleaning the parts in-situ and promoting excellent wetting by the braze filler.
Understanding the Trade-offs and Key Considerations
The Cost of Consumables
The primary trade-off is the operational cost. Nitrogen and especially argon are industrial consumables that must be purchased continuously. This cost must be factored into the price-per-part calculation.
However, this cost is almost always offset by the dramatic reduction in scrap, rework, and post-processing labor.
Furnace Integrity is Non-Negotiable
You can pump thousands of cubic feet of high-purity gas into a furnace, but if the chamber has leaks, you are simply wasting money. Outside air will be drawn in, contaminating the atmosphere.
Maintaining a well-sealed furnace with tight-fitting doors and properly maintained gaskets is essential for the process to be effective and efficient.
Matching the Gas to the Material
Not all atmospheres are suitable for all materials. For example, using a hydrogen-bearing atmosphere to braze certain steels can lead to hydrogen embrittlement, a catastrophic failure mechanism.
Always consult material and brazing specifications to select the correct atmosphere—whether pure inert, a nitrogen-hydrogen blend, or a vacuum—for your specific base metals.
Making the Right Choice for Your Goal
Selecting the proper atmosphere is a function of your desired outcome. Use these guidelines to inform your process decisions.
- If your primary focus is maximum joint strength: A pure, dry inert atmosphere is non-negotiable to ensure the filler metal flows completely and creates a bond free of voids and oxide inclusions.
- If your primary focus is process efficiency: The cost of an inert atmosphere is easily justified by the elimination of post-braze cleaning steps and the near-zero scrap rate from oxidation-related defects.
- If your primary focus is brazing stainless steel or other challenging alloys: An active atmosphere containing hydrogen is likely necessary to reduce tenacious surface oxides and ensure proper filler metal wetting.
Ultimately, controlling the furnace atmosphere is the single most important variable for controlling the quality and consistency of your final product.
Summary Table:
| Aspect | Key Details |
|---|---|
| Purpose | Prevents oxidation, ensures braze filler metal wetting and flow for strong joints |
| Common Gases | Nitrogen, Argon (inert); Nitrogen-Hydrogen blends (active) |
| Critical Factors | Low oxygen content, low moisture (dew point), furnace seal integrity |
| Benefits | Clean joints, reduced scrap, no post-braze cleaning, metallurgical soundness |
| Material Considerations | Avoid hydrogen embrittlement; match atmosphere to base metals (e.g., stainless steel requires active atmospheres) |
Ready to achieve flawless brazing results? At KINTEK, we specialize in advanced high-temperature furnace solutions tailored to your needs. Leveraging our exceptional R&D and in-house manufacturing, we offer products like Muffle, Tube, Rotary, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems, all with deep customization to meet your unique experimental requirements. Whether you're working with stainless steel, alloys, or other materials, our expertise in inert and active atmosphere control ensures strong, oxidation-free joints with maximum efficiency. Contact us today to discuss how we can enhance your brazing process and deliver reliable, high-quality outcomes!
Visual Guide
Related Products
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
People Also Ask
- How do argon and nitrogen protect samples in vacuum furnaces? Optimize Your Thermal Process with the Right Gas
- What are some specific applications of atmosphere furnaces in the ceramics industry? Enhance Purity and Performance
- Can box type high-temperature resistance furnaces control the atmosphere? Unlock Precision in Material Processing
- How does the pressure range change under vacuum conditions in an atmosphere box furnace? Explore Key Shifts for Material Processing
- What are the development prospects of atmosphere box furnaces in the aerospace industry? Unlock Advanced Material Processing for Aerospace Innovation



















