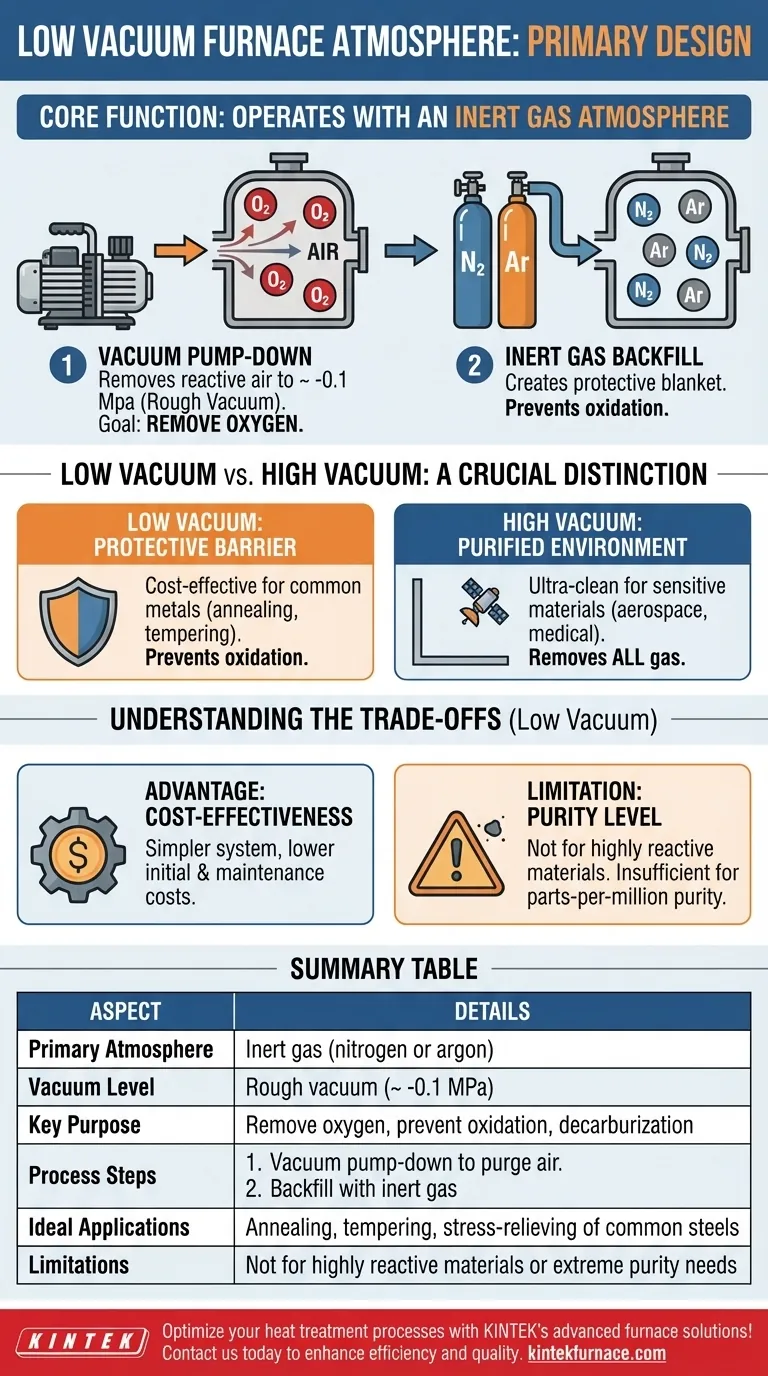
At its core, a low vacuum furnace is primarily designed to operate with an inert gas atmosphere, most commonly nitrogen or argon. The "vacuum" part of its name refers to the initial process of using a vacuum pump to remove reactive air before this inert gas is introduced, creating a controlled, low-oxygen environment.
The key purpose of a low vacuum furnace is not to create a true vacuum, but to efficiently purge oxygen and other reactive gases from the chamber. It then replaces this air with a protective, inert atmosphere to prevent oxidation during heat treatment.

The Role of the Atmosphere in a Low Vacuum Furnace
Understanding how this atmosphere is created reveals its function. The process is a deliberate two-step method designed for efficiency and protection.
Step 1: The Vacuum Pump-Down
The cycle begins by using a vacuum pump to remove the majority of the air inside the furnace chamber. This process typically achieves a vacuum level of around -0.1 Mpa, which is considered a rough or low vacuum.
The critical goal of this step is not to create an empty space, but to remove oxygen and other atmospheric gases that would react with the workpiece at high temperatures.
Step 2: The Inert Gas Backfill
Once the reactive air has been purged, the furnace is backfilled with a high-purity inert gas, such as nitrogen or argon.
This inert gas now fills the chamber, serving as a protective "blanket" around the material being processed. Because these gases do not readily react with other elements, they prevent oxidation, decarburization, and other unwanted surface reactions during the heating cycle.
Low Vacuum vs. High Vacuum: A Crucial Distinction
The term "vacuum furnace" can be confusing. The distinction between a low and high vacuum system is critical, as they serve different purposes.
Low Vacuum: A Protective Barrier
A low vacuum furnace uses its inert atmosphere as a protective barrier. The initial vacuum pull simply clears the way for this barrier to be effective. It is designed to be a cost-effective method for preventing the most common forms of atmospheric contamination, like oxidation.
High Vacuum: A Purified Environment
A high vacuum furnace, in contrast, aims to remove as many gas molecules as possible to create a near-perfectly empty and ultra-clean environment. This is essential for highly sensitive materials where even trace amounts of gas can cause contamination.
Understanding the Trade-offs
Choosing a low vacuum system involves a clear trade-off between cost and atmospheric purity.
Advantage: Cost-Effectiveness
Low vacuum furnaces are significantly less complex and expensive than their high vacuum counterparts. The pumps, seals, and control systems required are simpler, leading to lower initial investment and maintenance costs.
Limitation: Purity Level
A low vacuum system is not suitable for all applications. It does not create a truly pure environment. For materials that are extremely reactive or sensitive to even parts-per-million levels of contamination (like titanium or certain superalloys), a low vacuum is insufficient.
Consequence: Process Suitability
This makes low vacuum furnaces ideal for general heat-treating processes like annealing, tempering, and stress-relieving of common steels. The primary goal here is to prevent scale and heavy oxidation, which this system achieves perfectly.
Making the Right Choice for Your Goal
The correct furnace choice depends entirely on your material and the required outcome of the process.
- If your primary focus is preventing oxidation on common metals for processes like annealing: A low vacuum furnace backfilled with an inert gas provides excellent protection at a reasonable cost.
- If your primary focus is processing highly reactive materials or applications requiring extreme purity (e.g., medical, aerospace): A high vacuum furnace is non-negotiable to prevent any form of contamination.
Ultimately, matching the furnace's atmospheric capability to your specific process requirements is the key to achieving consistent, high-quality results.
Summary Table:
| Aspect | Details |
|---|---|
| Primary Atmosphere | Inert gas (nitrogen or argon) |
| Vacuum Level | Rough vacuum (~ -0.1 MPa) |
| Key Purpose | Remove oxygen, prevent oxidation, decarburization |
| Process Steps | 1. Vacuum pump-down to purge air 2. Backfill with inert gas |
| Ideal Applications | Annealing, tempering, stress-relieving of common steels |
| Limitations | Not for highly reactive materials or extreme purity needs |
Optimize your heat treatment processes with KINTEK's advanced furnace solutions! Leveraging exceptional R&D and in-house manufacturing, we provide diverse laboratories with high-temperature furnaces like Vacuum & Atmosphere Furnaces, tailored to your unique needs. Our deep customization ensures precise control for oxidation-free results. Contact us today to discuss how our products can enhance your efficiency and quality!
Visual Guide
Related Products
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
People Also Ask
- What are the benefits of inert atmosphere heat treating? Prevent Oxidation and Preserve Material Integrity
- What is the main purpose of heat treatment? Transform Metal Properties for Superior Performance
- How does nitrogen atmosphere heat treatment improve surface strengthening? Enhance Durability and Performance
- How does an inert atmosphere prevent oxidation? Shield Materials from Oxygen Damage
- How does a chemically inert atmosphere function in a furnace? Prevent Oxidation and Ensure Material Purity



















