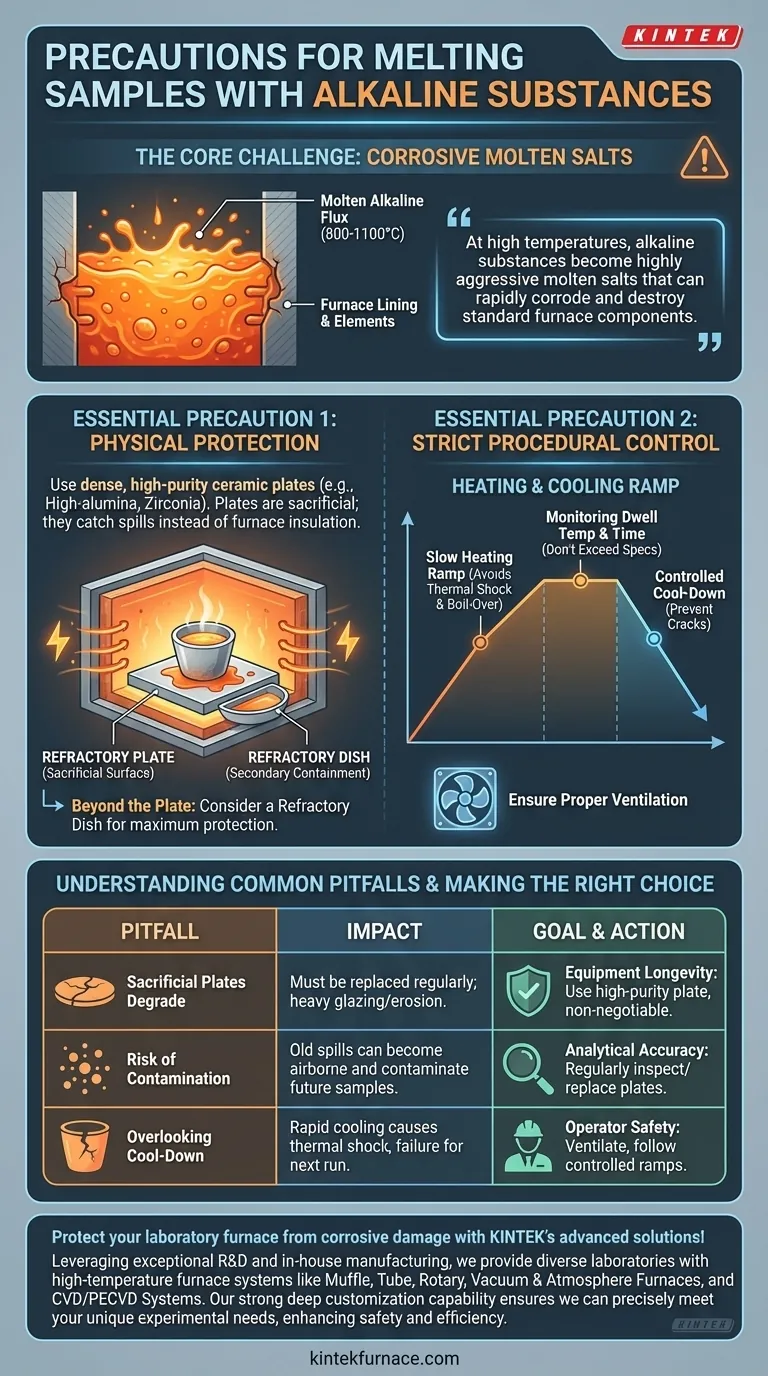
When melting samples with alkaline substances, you must implement both procedural controls and physical safeguards to protect your equipment and ensure accurate results. The most critical precautions are to strictly manage operating conditions—especially the heating rate—and to place a protective refractory plate at the bottom of the furnace to act as a barrier against corrosive spills.
At high temperatures, alkaline substances become highly aggressive molten salts that can rapidly corrode and destroy standard furnace components. The core strategy is therefore one of containment—using protective barriers and controlled heating to prevent any contact between the molten flux and the furnace itself.

The Core Challenge: Corrosive Molten Salts
The fundamental issue is not the heat alone, but the chemical reactivity of alkaline materials when they become molten. Understanding this is key to implementing effective precautions.
Why Molten Alkalis are so Aggressive
At fusion temperatures (often 800-1100°C), alkaline fluxes like lithium borate or sodium hydroxide transform into powerful solvents. They are especially effective at dissolving metal oxides, which are the primary components of most ceramics, including the furnace lining itself.
The Target of Corrosion: Furnace Linings and Elements
The interior of a high-temperature furnace is typically made of refractory bricks or ceramic fiber insulation. If a molten alkaline substance spills, it will chemically attack and eat through this insulation, potentially exposing and destroying the expensive heating elements and the furnace's metal shell.
Essential Precaution 1: Physical Furnace Protection
Your first line of defense is a physical barrier that isolates your sample crucible from the furnace floor.
The Role of the Refractory Plate
A refractory plate or hearth plate, placed on the furnace floor, serves as a sacrificial surface. Should your crucible crack or the sample boil over, the spill is caught by the plate, not the furnace insulation.
Material Selection is Critical
This plate must be made of a dense, high-purity ceramic that is resistant to your specific alkaline flux. High-alumina or zirconia plates are common choices. Using a low-quality or inappropriate material will result in the plate itself being rapidly corroded.
Beyond the Plate: Containing Spills
For maximum protection, consider placing your crucible inside a shallow refractory dish or tray. This provides secondary containment, ensuring that even a significant spill is fully captured before it has a chance to spread across the hearth plate.
Essential Precaution 2: Strict Procedural Control
Physical barriers can fail if the process itself is uncontrolled. How you heat and cool the sample is just as important as the protective hardware you use.
Controlling the Heating Rate
"Strictly controlling operating conditions" primarily refers to managing the temperature ramp rate. Heating the sample too quickly can cause two problems:
- Thermal Shock: The crucible may crack.
- Boil-Over: The flux may melt and outgas violently, bubbling over the side of the crucible.
A slow, programmed heating ramp is essential to allow for gentle melting and reaction.
Monitoring Dwell Temperature and Time
Do not exceed the necessary temperature or time specified by your analytical method. Higher temperatures dramatically increase the corrosivity of molten salts and the risk of component failure.
Ensuring Proper Ventilation
Melting and fusion processes can release fumes. Always operate the furnace in a well-ventilated area or under a fume hood to ensure operator safety.
Understanding the Common Pitfalls
Even with precautions, mistakes can happen. Being aware of them is critical for long-term success and safety.
Sacrificial Plates Are Not Permanent
The hearth plate is a consumable item. After catching a spill, or even after prolonged exposure to the high-temperature environment, it will degrade. It must be inspected regularly and replaced when it shows signs of cracking, heavy glazing, or erosion.
The Risk of Contamination
A degraded hearth plate can become a source of contamination. If material from a previous spill is not fully cleaned or the plate itself begins to break down, its particles can become airborne within the furnace and contaminate future samples, compromising analytical accuracy.
Overlooking Cool-Down Procedures
Just as a slow heating ramp is critical, a controlled cool-down is equally important. Cooling the furnace too quickly can induce thermal shock, cracking both the crucible and the protective hearth plate, setting you up for failure on the next run.
Making the Right Choice for Your Goal
Your specific approach should align with your primary objective. Use these guidelines to prioritize your actions.
- If your primary focus is equipment longevity: Always use a high-purity, correctly sized refractory hearth plate as a non-negotiable first line of defense.
- If your primary focus is analytical accuracy: Regularly inspect your protective plates and crucibles for any signs of degradation to prevent cross-contamination between samples.
- If your primary focus is operator safety: Ensure the furnace is in a well-ventilated area and always follow a slow, controlled heating and cooling ramp to prevent spills and thermal shock.
By treating molten alkalis with the respect they command, you safeguard your process, your equipment, and your results.
Summary Table:
| Precaution Type | Key Actions | Purpose |
|---|---|---|
| Physical Protection | Use refractory plates and dishes | Contain spills and protect furnace linings |
| Procedural Control | Manage heating rates and ventilation | Prevent boil-over and ensure operator safety |
| Maintenance | Regularly inspect and replace plates | Avoid contamination and equipment failure |
Protect your laboratory furnace from corrosive damage with KINTEK's advanced solutions! Leveraging exceptional R&D and in-house manufacturing, we provide diverse laboratories with high-temperature furnace systems like Muffle, Tube, Rotary, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems. Our strong deep customization capability ensures we can precisely meet your unique experimental needs, enhancing safety and efficiency. Contact us today to discuss how we can support your work with reliable, tailored equipment!
Visual Guide
Related Products
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Molybdenum Vacuum Heat Treat Furnace
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
People Also Ask
- What role does a high-temperature vacuum heat treatment furnace play in TBC post-processing? Enhance Coating Adhesion
- What are the benefits of vacuum heat treatment? Achieve Superior Metallurgical Control
- What are the functions of a high-vacuum furnace for CoReCr alloys? Achieve Microstructural Precision and Phase Stability
- What is the vacuum heat treatment process? Achieve Superior Surface Quality and Material Performance
- Why does heating steel rod bundles in a vacuum furnace eliminate heat transfer paths? Enhance Surface Integrity Today



















