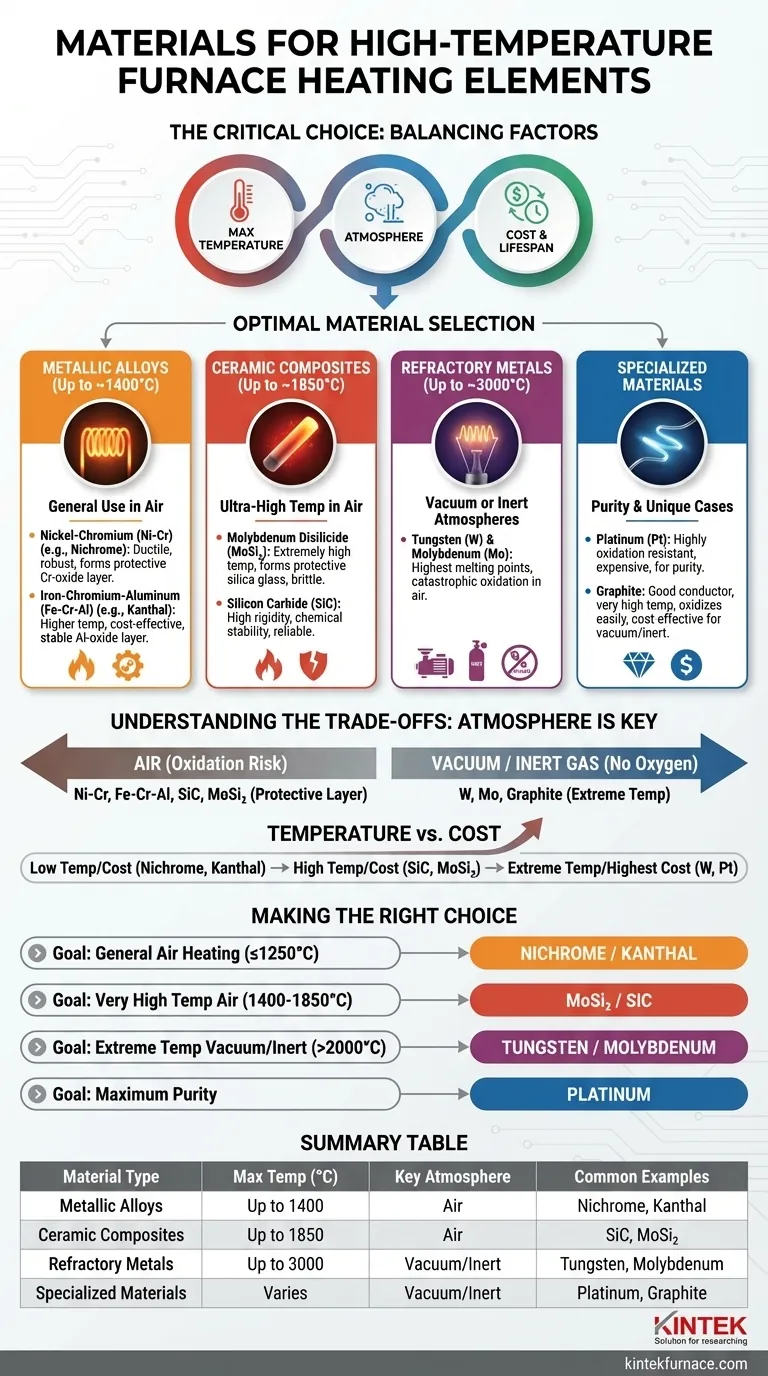
The choice of material for a high-temperature furnace heating element is dictated by its maximum operating temperature and, most critically, the atmosphere in which it will operate. Common materials are grouped into metallic alloys like Nichrome and Kanthal for general use, ceramic composites like silicon carbide (SiC) and molybdenum disilicide (MoSi₂) for very high temperatures in air, and refractory metals like tungsten and molybdenum for the highest temperatures achievable in vacuum or inert atmospheres.
Selecting a heating element is not just about finding a material that gets hot enough. It is a critical engineering decision that balances the required temperature, the furnace's operating atmosphere, material cost, and the element's expected lifespan. The correct choice depends entirely on matching the material's properties to the specific application's demands.

The Foundation: Why These Materials Work
The function of a heating element is to convert electrical energy into heat through resistance. To do this effectively and durably at high temperatures, a material must possess a few non-negotiable properties.
High Electrical Resistivity
A material with high resistance generates significant heat (Joule heating) when electrical current passes through it, without requiring excessively long wires. This allows for compact and efficient furnace designs.
High Melting Point
This is the most obvious requirement. The element's material must remain solid and structurally stable well above the furnace's maximum operating temperature.
Resistance to Oxidation and Corrosion
At high temperatures, most materials react rapidly with oxygen in the air, leading to degradation and failure. The best heating elements either form a stable, protective oxide layer on their surface or are used in an environment completely free of oxygen.
A Breakdown of Common High-Temperature Materials
Heating element materials are best understood by grouping them into distinct classes, each suited for different temperature ranges and operating environments.
Metallic Alloys: The Workhorses (Up to ~1400°C)
These alloys are the most common choice for industrial and laboratory furnaces operating in air.
- Nickel-Chromium (Ni-Cr) Alloys (e.g., Nichrome): Typically an 80/20 mix of nickel and chromium, this is the classic heating element material. It is ductile, robust, and forms a protective layer of chromium oxide that prevents further corrosion in air.
- Iron-Chromium-Aluminum (Fe-Cr-Al) Alloys (e.g., Kanthal): These alloys can reach slightly higher temperatures than Nichrome and are often more cost-effective. They form a very stable aluminum oxide layer that offers excellent protection.
Ceramic Composites: The Ultra-High Temperature Champions (Up to ~1850°C)
When temperatures in an air-filled furnace need to exceed the limits of metallic alloys, ceramic-based elements are required.
- Molybdenum Disilicide (MoSi₂): These elements can operate at extremely high temperatures in air because they form a protective silica glass layer. They are, however, brittle at room temperature and require careful handling.
- Silicon Carbide (SiC): Known for its high rigidity and excellent chemical stability, SiC is a reliable choice for high-temperature applications. It functions well in air and various controlled atmospheres.
Refractory Metals: For Vacuum Environments (Up to ~3000°C)
Refractory metals have the highest melting points of all materials but share a critical weakness: they oxidize catastrophically in air at high temperatures.
- Tungsten (W) and Molybdenum (Mo): These are the go-to materials for vacuum furnaces or those filled with an inert gas (like argon). Tungsten boasts the highest melting point of any metal, enabling the most extreme temperature applications, but it cannot be exposed to oxygen when hot.
Specialized Materials: For Purity and Unique Cases
- Platinum (Pt): While extremely expensive, platinum is highly resistant to oxidation and does not contaminate the furnace environment. It is used in specialized applications where product purity is the absolute priority.
- Graphite: A good conductor that can withstand very high temperatures, but like refractory metals, it oxidizes easily. It is a cost-effective option for heating elements in vacuum or inert-gas furnaces.
Understanding the Trade-offs: Atmosphere is Everything
The decision-making process is a series of trade-offs, with the furnace's internal atmosphere being the most significant factor.
Air vs. Vacuum/Inert Gas
This is the primary dividing line. If your process occurs in open air, your choices are limited to materials that form a protective oxide layer, such as Ni-Cr, Fe-Cr-Al, SiC, and MoSi₂. If you use a vacuum or inert gas, you can use Tungsten, Molybdenum, or Graphite, which offer higher temperature capabilities but require a more complex and sealed furnace system.
Temperature vs. Cost
There is a direct correlation between maximum operating temperature and cost. Nichrome and Kanthal alloys are the most economical for moderate high-temperature work. SiC and MoSi₂ represent a significant step up in both temperature capability and price. Tungsten and Platinum sit at the highest end of the cost spectrum, reserved for applications where their unique properties are indispensable.
Brittleness and Durability
Metallic alloys like Nichrome are ductile and resistant to mechanical shock. In contrast, ceramic elements like SiC and MoSi₂ are brittle, especially at lower temperatures, and can be susceptible to thermal shock if heated or cooled too quickly.
Making the Right Choice for Your Furnace
Your optimal material depends directly on your primary operational goal.
- If your primary focus is general-purpose heating in air up to 1250°C: Nichrome or Kanthal alloys offer the best balance of performance, durability, and cost.
- If your primary focus is very high-temperature operation in air (1400°C - 1850°C): Molybdenum disilicide (MoSi₂) or silicon carbide (SiC) elements are necessary to withstand these conditions.
- If your primary focus is achieving extreme temperatures in a vacuum or inert atmosphere: Tungsten or molybdenum are the only practical choices for reliable performance above 2000°C.
- If your primary focus is preventing any material contamination at high temperatures: Platinum is the ideal, albeit most expensive, solution for maintaining a pure furnace environment.
By understanding this interplay between material, atmosphere, and temperature, you can select a heating element that ensures reliable, efficient, and long-lasting performance for your specific needs.
Summary Table:
| Material Type | Max Temperature (°C) | Key Atmosphere | Common Examples |
|---|---|---|---|
| Metallic Alloys | Up to 1400 | Air | Nichrome, Kanthal |
| Ceramic Composites | Up to 1850 | Air | Silicon Carbide (SiC), Molybdenum Disilicide (MoSi₂) |
| Refractory Metals | Up to 3000 | Vacuum/Inert | Tungsten, Molybdenum |
| Specialized Materials | Varies | Vacuum/Inert | Platinum, Graphite |
Ready to optimize your high-temperature furnace with the perfect heating element? At KINTEK, we leverage exceptional R&D and in-house manufacturing to provide advanced solutions like Muffle, Tube, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems. Our strong deep customization capability ensures we meet your unique experimental needs precisely. Contact us today to discuss how we can enhance your lab's performance and reliability!
Visual Guide
Related Products
- Silicon Carbide SiC Thermal Heating Elements for Electric Furnace
- Molybdenum Vacuum Heat Treat Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
People Also Ask
- Why are SIC heating elements resistant to chemical corrosion? Discover the Self-Protecting Mechanism
- What makes SIC heating elements superior for high-temperature applications? Unlock Efficiency and Durability
- Why is silicon carbide resistant to chemical reactions in industrial furnaces? Unlock Durable High-Temp Solutions
- Why are silicon carbide heating elements essential in high-temperature industries? Unlock Reliable, Extreme Heat Solutions
- Why are SiC heating elements considered environmentally friendly? Discover Their Eco-Efficiency & Lifespan Insights



















