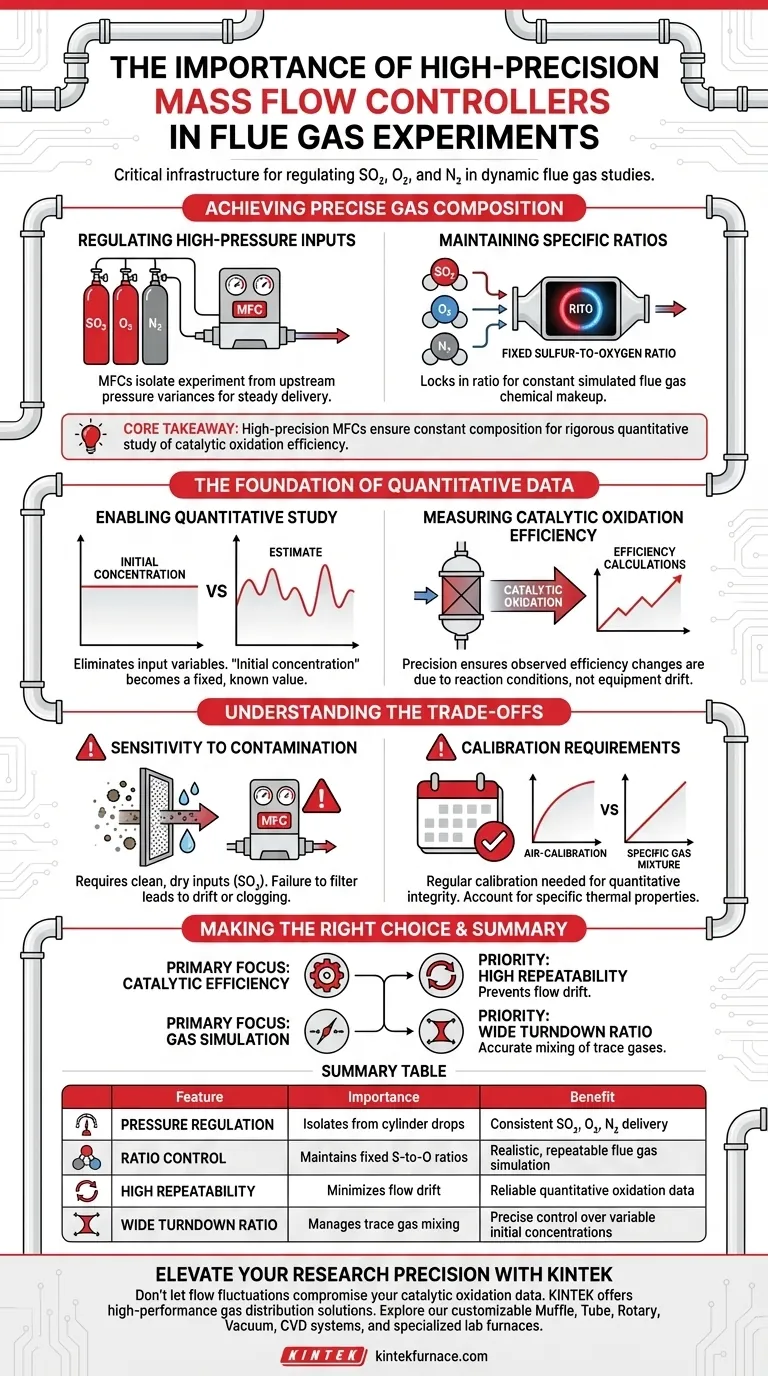
High-precision mass flow controllers (MFCs) are the critical infrastructure for valid flue gas experiments. They provide the exact regulation and mixing of high-pressure experimental gases—specifically SO2, O2, and N2—required to create a stable, simulated flue gas environment. Without this level of control, maintaining the constant composition ratios necessary for accurate research becomes impossible.
Core Takeaway: In dynamic flue gas studies, the validity of your data relies on the stability of your input. High-precision MFCs ensure a constant gas composition, allowing for the rigorous quantitative study of catalytic oxidation efficiency without the interference of fluctuating gas concentrations.

Achieving Precise Gas Composition
Regulating High-Pressure Inputs
Experimental gases such as Sulfur Dioxide (SO2), Oxygen (O2), and Nitrogen (N2) are typically delivered at high pressures.
High-precision controllers are essential to regulate these flows accurately. They isolate the experiment from upstream pressure variances, ensuring the delivery rate remains steady regardless of cylinder conditions.
Maintaining Specific Ratios
The primary goal in these experiments is often to simulate a specific sulfur-to-oxygen ratio.
Standard flow meters may allow for significant variance. High-precision MFCs lock this ratio in, ensuring the simulated flue gas maintains a constant chemical makeup throughout the entire process.
The Foundation of Quantitative Data
Enabling Quantitative Study
To understand how initial gas concentrations influence results, you must eliminate input variables.
High-precision MFCs provide the certainty required for quantitative study. They ensure that the "initial concentration" variable in your data set is a fixed, known value rather than an estimate.
Measuring Catalytic Oxidation Efficiency
A major application of this technology is determining the catalytic oxidation efficiency of SO2.
Efficiency calculations are highly sensitive to input flow. If the reactant flow rate drifts, the calculated efficiency will be skewed. Precision control ensures that observed changes in efficiency are due to the reaction conditions, not equipment error.
Understanding the Trade-offs
Sensitivity to Contamination
High-precision instruments are often more sensitive to particulate matter or moisture than robust, lower-precision rotameters.
When using reactive gases like SO2, the gas source must be clean and dry. Failure to filter inputs can lead to sensor drift or clogging, negating the benefits of the high-precision device.
Calibration Requirements
Accuracy is not permanent. To maintain the quantitative integrity of your study, these controllers require regular calibration.
You must account for the specific thermal properties of the gas mixture (especially when mixing N2 and SO2), as standard air-calibration curves may not provide the necessary accuracy for these specific experimental gases.
Making the Right Choice for Your Experiment
To ensure your setup meets your experimental goals, consider the following:
- If your primary focus is Catalytic Efficiency: Prioritize controllers with high repeatability to ensure that calculated oxidation rates are not skewed by flow drift.
- If your primary focus is Gas Simulation: Ensure the controllers have a wide turndown ratio to accurately mix trace gases (like SO2) with bulk gases (like N2).
Precision in your flow control is the only way to guarantee confidence in your reaction data.
Summary Table:
| Feature | Importance in Flue Gas Experiments | Benefit to Researcher |
|---|---|---|
| Pressure Regulation | Isolates system from cylinder pressure drops | Consistent delivery of SO2, O2, and N2 |
| Ratio Control | Maintains fixed sulfur-to-oxygen ratios | Realistic and repeatable flue gas simulation |
| High Repeatability | Minimizes flow drift during long tests | Reliable quantitative oxidation efficiency data |
| Wide Turndown Ratio | Manages trace gas mixing with bulk N2 | Precise control over variable initial concentrations |
Elevate Your Research Precision with KINTEK
Don’t let flow fluctuations compromise your catalytic oxidation data. KINTEK provides high-performance gas distribution solutions designed to meet the rigorous demands of laboratory research.
Backed by expert R&D and manufacturing, KINTEK offers a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems, along with specialized lab high-temp furnaces—all fully customizable to your unique flue gas simulation needs. Whether you are studying SO2 oxidation or complex gas-solid reactions, our equipment ensures the thermal and flow stability your work deserves.
Ready to optimize your experimental setup? Contact our technical experts today to find the perfect precision solution for your laboratory.
Visual Guide
References
- Haipeng Liu, Hongying Yang. Generation and Inhibition of SO3 in Lead Smelting Flue Gas. DOI: 10.3390/app15084449
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- Stainless Steel Quick Release Vacuum Chain Three Section Clamp
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- Multi Heating Zones CVD Tube Furnace Machine for Chemical Vapor Deposition Equipment
- Vacuum Hot Press Furnace Machine for Lamination and Heating
People Also Ask
- What necessary conditions does a vacuum chamber provide for vapor deposition? Achieve High-Purity Nanofluid Synthesis
- What are the advantages of using a single-mode microwave generator? Precision Heating for Metal Recovery
- Why use high-purity graphite for β-Ga2O3 annealing? Key to Thermal Precision & Safety
- Why is it necessary to achieve a vacuum level of 3 x 10^-2 mm Hg for quartz tube sealing? Ensure Safety and Purity
- What is the function of high-purity alumina crucibles? Protect Samples and Furnaces During Oxide Calcination
- Why is ultrasonic cleaning with acetone required before thermal oxidation? Ensure Perfect Stainless Steel Adhesion
- Why are magnesium oxide-stabilized zirconia crucibles used for melting alloys? High-Temp Stability up to 1900°C
- How does a precision Mass Flow Controller (MFC) regulate argon carrier gas to affect the growth of WS2 nanosheets?








