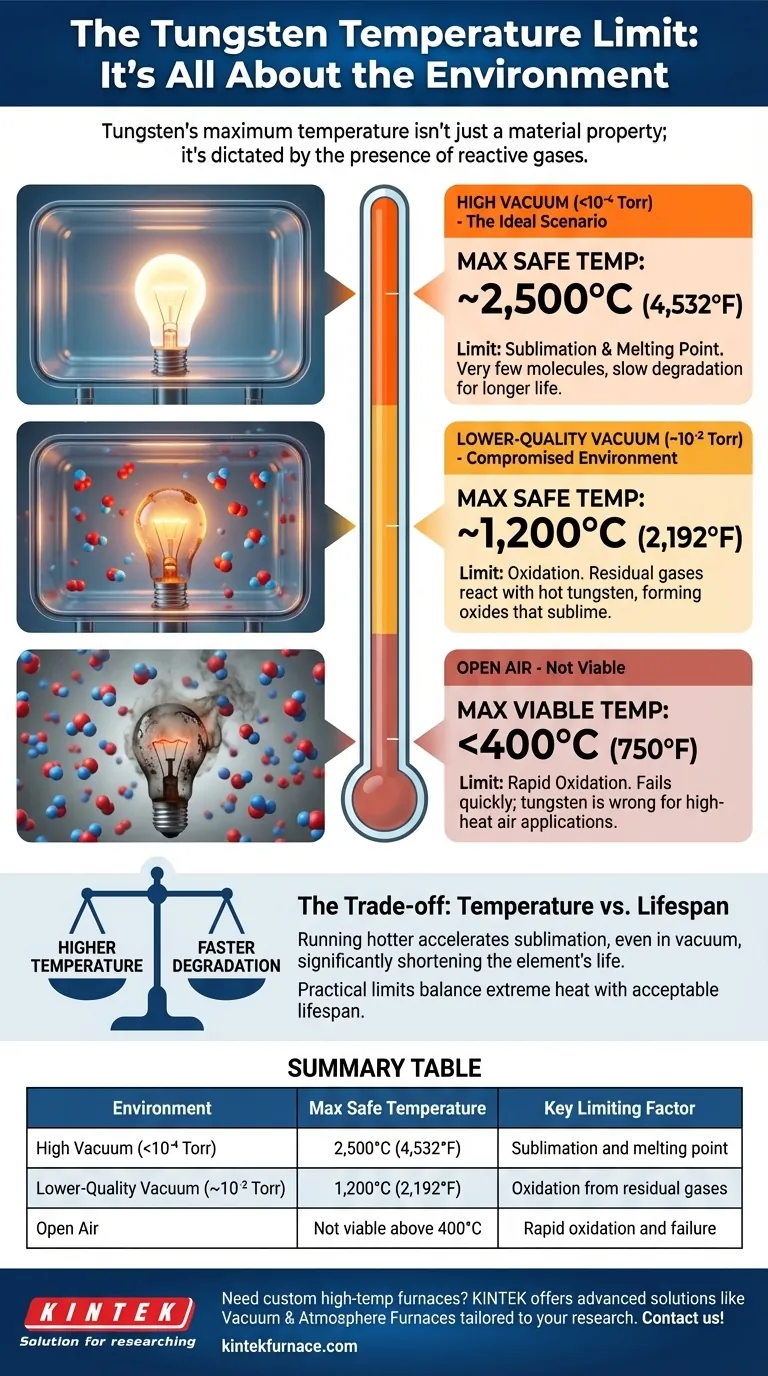
The maximum temperature a tungsten heating element can reach is entirely dependent on its operating environment. While theoretically capable of reaching over 6,100°F (around 3,400°C) in a perfect vacuum, its practical and safe operating temperature is significantly lower and dictated by the presence of oxygen and other reactive gases.
The true limit of a tungsten element isn't just its high melting point, but its extreme vulnerability to oxidation. Its maximum usable temperature is therefore a direct function of the quality of the vacuum or inert atmosphere it operates within.

Why Environment is the Deciding Factor
Tungsten's remarkable heat resistance is only half the story. To understand its real-world limits, you must first understand how it interacts with its surroundings at extreme temperatures.
In a High Vacuum: The Ideal Scenario
Under a high vacuum (less than 10⁻⁴ Torr), there are very few gas molecules to react with the hot tungsten. This is the ideal condition, allowing the element to safely reach sustained temperatures around 4,532°F (2,500°C). The ultimate limit here is tungsten's melting point of 6,192°F (3,422°C), but operating that close significantly shortens the element's lifespan.
In a Lower-Quality Vacuum
As the vacuum quality degrades (e.g., to 10⁻² Torr), more residual oxygen and water vapor are present. These molecules will react with the tungsten, limiting the safe maximum temperature to around 2,192°F (1,200°C). Pushing beyond this in a poor vacuum will cause rapid degradation and premature failure.
In Open Air: The Non-Starter
Using a tungsten heating element in open air is not viable for high-temperature applications. The tungsten will begin to rapidly oxidize at temperatures as low as 750°F (400°C). It will quickly burn out, producing tungsten oxide and failing completely long before it reaches its potential.
Understanding the Trade-offs: Temperature vs. Lifespan
Choosing an operating temperature is always a balance between performance and longevity. The primary factor you are fighting against is the degradation of the element itself.
The Problem of Oxidation
Oxidation is the primary enemy of a hot tungsten element. When tungsten atoms react with oxygen, they form tungsten oxide. This oxide has a much lower boiling point than the metal itself, causing it to "boil off" or sublimate from the surface of the element. This process thins the element until it breaks.
Balancing Heat and Sublimation
Even in a perfect vacuum, running an element near its melting point causes the tungsten itself to sublimate, or turn directly from a solid into a gas. The hotter the element, the faster this happens. Therefore, the practical maximum temperature (like 2,500°C) is a compromise designed to provide extreme heat while ensuring an acceptable operational lifespan.
Making the Right Choice for Your Goal
The correct application of a tungsten element requires matching your temperature goal to the appropriate environment.
- If your primary focus is achieving maximum heat (2,000°C+): You must invest in a high-vacuum furnace or chamber (below 10⁻⁴ Torr) to prevent oxidation.
- If your primary focus is moderate heat in a less-controlled environment: A lower-grade vacuum or an inert gas backfill (like Argon) is necessary to reach temperatures up to approximately 1,200°C.
- If your primary focus is heating in open air: Tungsten is the wrong material; you should select an element designed for oxidative atmospheres, such as Kanthal (FeCrAl) or Nichrome (NiCr).
Ultimately, harnessing the power of tungsten requires controlling its environment with precision.
Summary Table:
| Environment | Maximum Safe Temperature | Key Limiting Factor |
|---|---|---|
| High Vacuum (<10⁻⁴ Torr) | 2,500°C (4,532°F) | Sublimation and melting point |
| Lower-Quality Vacuum (~10⁻² Torr) | 1,200°C (2,192°F) | Oxidation from residual gases |
| Open Air | Not viable above 400°C (750°F) | Rapid oxidation and failure |
Need a high-temperature furnace tailored to your lab's unique needs? At KINTEK, we leverage exceptional R&D and in-house manufacturing to provide advanced solutions like Muffle, Tube, Rotary, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems. Our strong deep customization capability ensures precise alignment with your experimental requirements, whether you're pushing limits in high-vacuum environments or optimizing for moderate heat. Contact us today to discuss how our tungsten heating elements and furnaces can enhance your research and efficiency!
Visual Guide
Related Products
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Laboratory Muffle Oven Furnace with Bottom Lifting
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
People Also Ask
- Why might a vacuum furnace maintain vacuum during cooling? Protect Workpieces from Oxidation and Control Metallurgy
- How do vacuum furnaces contribute to long-term cost savings? Reduce Costs with Efficiency and Quality
- What is the function of a vacuum sintering furnace in the SAGBD process? Optimize Magnetic Coercivity and Performance
- How are parts loaded into a vacuum furnace? Ensure Precision and Efficiency in Your Process
- How do vacuum sintering and annealing furnaces contribute to the densification of NdFeB magnets?



















