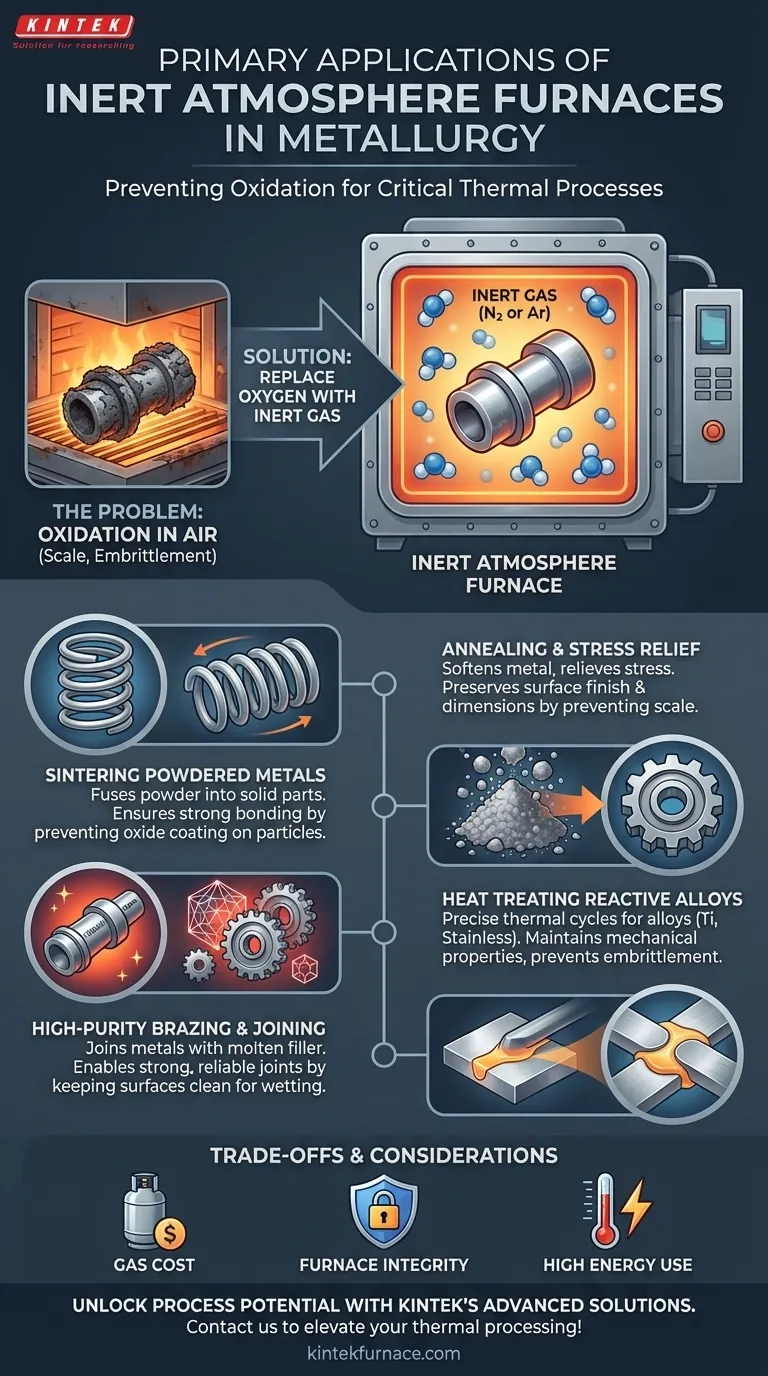
In short, inert atmosphere furnaces are used in metallurgy for critical thermal processes where preventing oxidation is paramount. Their primary applications include the annealing of metals to relieve stress, the sintering of powdered metals to form solid parts, and the specialized heat treatment (like hardening or tempering) of highly reactive alloys such as titanium and stainless steel.
The core purpose of an inert atmosphere furnace is not just to heat a material, but to create a chemically neutral environment. This ensures the material's final properties are the direct result of the intended thermal process, completely unaltered by unwanted chemical reactions with oxygen in the air.

Why an Inert Atmosphere is Critical
At the high temperatures required for most metallurgical treatments, metals become highly reactive with the oxygen present in ambient air. This reaction, oxidation, fundamentally changes the material you are trying to process.
The Problem: Oxidation at High Temperatures
When heated in air, most metals form a brittle, flaky layer of oxide on their surface. This "scale" can ruin surface finish, compromise dimensional tolerances, and, more critically, alter the mechanical properties of the component.
For reactive metals like titanium or certain high-strength steels, oxygen can diffuse into the material itself, causing embrittlement and dramatically reducing its strength and fatigue life.
The Solution: Replacing Oxygen with an Inert Gas
An inert atmosphere furnace solves this by purging all the air from its sealed chamber and replacing it with a non-reactive gas.
Nitrogen (N₂) is the most common and cost-effective choice, as it does not react with most steels. For more reactive metals that can form nitrides (like titanium), a truly inert noble gas like Argon (Ar) is used. This creates a stable environment where heat can be applied without causing unwanted chemical changes.
Key Metallurgical Applications in Detail
Using this controlled environment enables several high-precision processes that would otherwise be impossible or result in inferior products.
Annealing and Stress Relief
The goal of annealing is to soften a metal and relieve internal stresses built up during forming processes. An inert atmosphere ensures this process happens without creating a hard oxide scale on the surface, preserving the part's finish and dimensions.
Sintering of Powdered Metals
Sintering involves heating compacted metal powders to just below their melting point, causing the particles to fuse into a solid, dense object. If oxygen were present, it would coat every powder particle, preventing them from bonding properly and resulting in a weak, porous, and useless part.
Heat Treating Reactive Alloys
Processes like hardening, case hardening, and tempering rely on precise thermal cycles to achieve specific microstructures and mechanical properties. For alloys prone to oxidation, performing these treatments in air would simultaneously weaken the material you are trying to strengthen. An inert atmosphere is essential for processing aerospace-grade titanium, medical implants, and high-performance stainless steel components.
High-Purity Brazing and Joining
Brazing joins two pieces of metal using a molten filler material. For a strong joint, the filler must "wet" the surfaces of the base metals completely. An oxide layer prevents this, leading to a weak or incomplete bond. An inert atmosphere ensures the metal surfaces remain perfectly clean, allowing for a strong, reliable, and continuous joint.
Understanding the Trade-offs
While essential for high-performance applications, inert atmosphere furnaces come with specific operational challenges that must be managed.
The Cost of Inert Gas
The continuous consumption of high-purity nitrogen or argon represents a significant operational cost. This is a primary factor to consider when evaluating the necessity of an inert process versus a less pure alternative.
Furnace Integrity and Maintenance
To be effective, the furnace chamber must be hermetically sealed. Any leak will introduce oxygen and compromise the entire process. This requires robust engineering, careful monitoring, and diligent maintenance to ensure seals and gaskets remain intact.
High Energy Consumption
Reaching and maintaining the elevated temperatures for many of these processes (often exceeding 1000°C) requires a substantial amount of energy, adding to the overall cost of operation.
The Alternative: Vacuum Furnaces
It's important to note that vacuum furnaces achieve the same goal—removing oxygen—but by pumping the atmosphere out rather than replacing it. For materials where even nitrogen is too reactive, a high vacuum is often the preferred choice. For less sensitive parts, a "low vacuum" furnace can be a cost-effective compromise between a full inert atmosphere and processing in open air.
Making the Right Choice for Your Process
The decision to use an inert atmosphere furnace depends entirely on the material being processed and the desired quality of the final product.
- If your primary focus is surface finish and preventing any discoloration: An inert atmosphere is non-negotiable for achieving a bright, clean surface on parts that require no post-processing.
- If your primary focus is the bulk mechanical properties of highly reactive metals (like titanium): An inert or vacuum environment is essential to prevent internal embrittlement and ensure the heat treatment delivers the intended strength.
- If your primary focus is cost-efficiency for less sensitive materials (like some steels): You may be able to use a lower-purity atmosphere or even a low-vacuum furnace, balancing cost against acceptable levels of surface oxidation.
Ultimately, selecting an inert atmosphere furnace is a strategic decision to control a material's chemistry at high temperatures, guaranteeing the integrity and performance of your final product.
Summary Table:
| Application | Key Benefit |
|---|---|
| Annealing and Stress Relief | Preserves surface finish and dimensions by preventing oxide scale |
| Sintering of Powdered Metals | Ensures strong, dense parts by allowing proper particle bonding |
| Heat Treating Reactive Alloys | Maintains mechanical properties and prevents embrittlement |
| High-Purity Brazing and Joining | Enables strong, reliable joints by keeping metal surfaces clean |
Unlock the full potential of your metallurgical processes with KINTEK's advanced inert atmosphere furnaces! Leveraging exceptional R&D and in-house manufacturing, we provide diverse laboratories with high-temperature solutions like Muffle, Tube, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems. Our strong deep customization capability ensures precise alignment with your unique experimental needs, enhancing efficiency and product quality. Contact us today to discuss how we can elevate your thermal processing!
Visual Guide
Related Products
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
People Also Ask
- How does the pressure range change under vacuum conditions in an atmosphere box furnace? Explore Key Shifts for Material Processing
- What are the key features of an atmosphere box furnace? Unlock Precise Heat Processing in Controlled Environments
- How do argon and nitrogen protect samples in vacuum furnaces? Optimize Your Thermal Process with the Right Gas
- What is an atmosphere protection muffle furnace? Unlock Precise Heat Treatment in Controlled Environments
- What are some specific applications of atmosphere furnaces in the ceramics industry? Enhance Purity and Performance



















