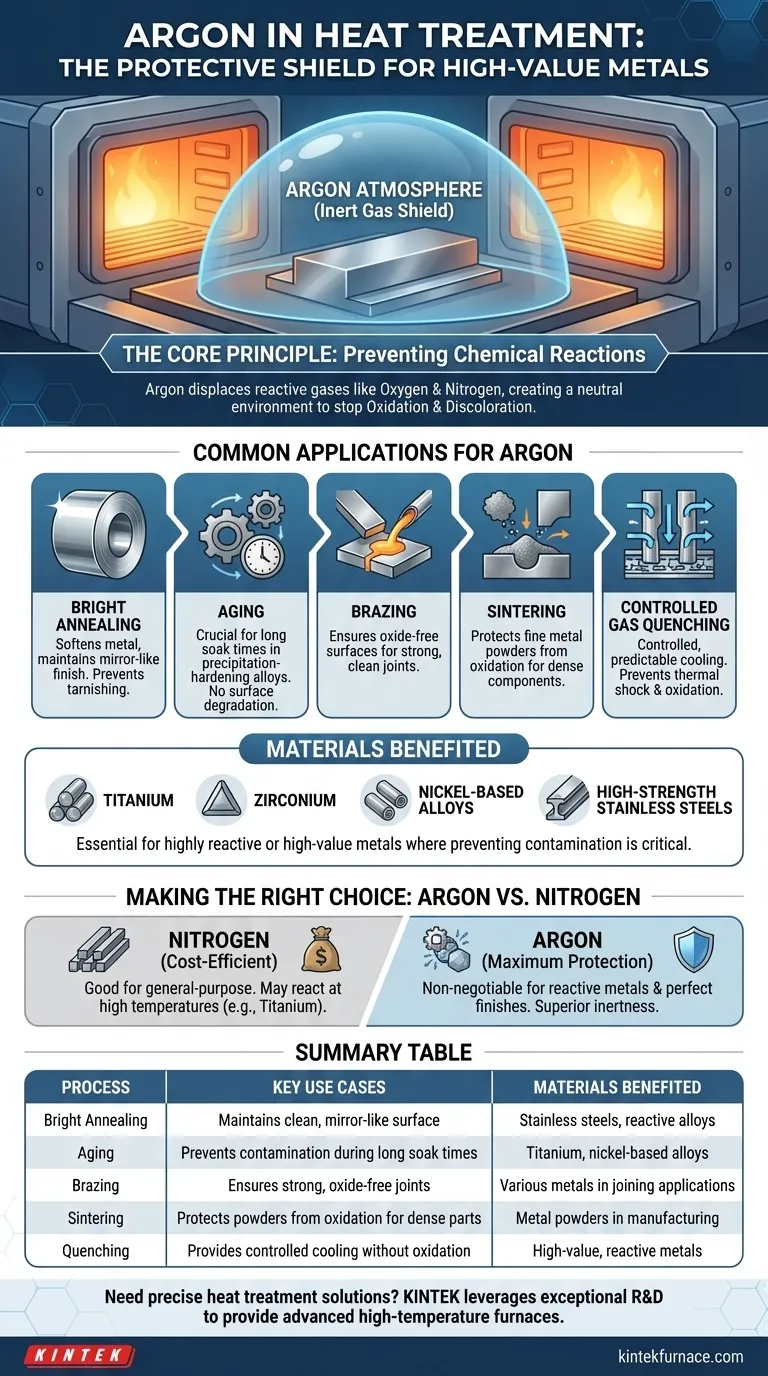
In heat treatment, argon is most commonly used in processes where preventing any chemical reaction with the workpiece is critical. These include bright annealing, aging, brazing, sintering, and quenching, especially when working with highly reactive or high-value metals like titanium, zirconium, and certain high-strength stainless steels.
The decision to use argon is not about the specific process, but about the material's sensitivity. Its fundamental value is its chemical inertness, which creates a protective shield that prevents oxygen and other atmospheric gases from damaging the metal at high temperatures.

The Core Principle: Why Inert Gas is Critical
Heat treatment relies on precise temperature control to alter a material's physical and mechanical properties. However, high temperatures also accelerate chemical reactions, primarily with gases present in the air.
The Problem of a Reactive Atmosphere
At elevated temperatures, metals become highly susceptible to reacting with oxygen, nitrogen, and water vapor. This can lead to oxidation (scaling), discoloration, and the formation of undesirable compounds like nitrides.
These reactions don't just affect the surface appearance; they can fundamentally change the material's properties, compromising its strength, corrosion resistance, and structural integrity.
Argon's Role as a Protective Shield
Argon is a noble gas, meaning it is chemically inert. It does not react with other elements, even under the intense conditions of a heat treatment furnace.
By flooding the furnace chamber with argon, you displace the reactive atmospheric gases. This creates a completely neutral environment, ensuring the material is only affected by the heat and not by any unwanted chemical changes.
Common Heat Treatment Applications for Argon
Argon's protective qualities make it essential for several specific processes, particularly when the material's final properties and surface finish are paramount.
Annealing and Aging
In bright annealing, the goal is to soften a metal and relieve internal stresses while maintaining a clean, mirror-like surface. Argon prevents the oxidation that would otherwise tarnish the finish.
For aging heat treatments on precipitation-hardening alloys (like titanium and nickel-based alloys), argon is crucial. These processes require holding a material at a precise temperature for extended periods. Argon ensures no contamination or surface degradation occurs during this long soak time.
Brazing and Sintering
Brazing joins two pieces of metal using a molten filler material. An argon atmosphere prevents oxides from forming on the base metals, which would inhibit the filler metal from wetting the surface and creating a strong, clean joint.
In sintering, fine metal powders are heated until they bond together to form a solid part. Argon protects these tiny particles from oxidation, ensuring a dense, strong final component.
Treating Highly Reactive Metals
For industries like aerospace and medical devices, argon is non-negotiable. Materials like titanium, zirconium, and other high-performance alloys are extremely reactive at high temperatures.
Exposing them to air would cause them to absorb gases, leading to embrittlement and catastrophic failure. Argon's inert shield is the only way to guarantee these materials retain their specified strength and durability.
Controlled Gas Quenching
While less common than liquid quenching, gas quenching in an argon atmosphere provides a controlled, predictable cooling rate. It prevents both oxidation during the cool-down phase and the potential for thermal shock or distortion that can occur with liquids.
Understanding the Trade-offs
While argon offers superior protection, it's essential to understand its place relative to other atmospheric gases, primarily nitrogen.
Cost vs. Performance: Argon vs. Nitrogen
Nitrogen is the most common furnace atmosphere because it is relatively inert and significantly less expensive than argon. For many general-purpose heat treatments on standard carbon and alloy steels, a nitrogen atmosphere is perfectly adequate.
However, nitrogen is not truly inert. At very high temperatures, it can react with certain metals—most notably titanium—to form hard, brittle nitrides. This is why argon is the mandatory choice for highly reactive materials.
Atmosphere Purity and Furnace Integrity
The effectiveness of an argon shield depends entirely on eliminating reactive gases. This means using high-purity argon and ensuring the furnace is well-sealed.
Any leaks that allow air to enter the chamber will contaminate the atmosphere, negating the benefits and cost of using argon in the first place.
Making the Right Choice for Your Goal
Selecting the correct furnace atmosphere is a balance between material requirements, process goals, and cost.
- If your primary focus is cost-efficiency for non-reactive metals: A nitrogen-based atmosphere is often the most practical choice for general-purpose heat treating of standard steels.
- If your primary focus is treating reactive metals (titanium, zirconium, nickel alloys): Argon is non-negotiable to prevent the formation of detrimental nitrides or oxides and preserve mechanical integrity.
- If your primary focus is a perfect surface finish (bright annealing) or maximum joint purity (brazing): Argon provides the highest level of protection against any surface discoloration or contamination.
Ultimately, choosing argon is an investment in process stability, guaranteeing the material properties and surface quality are preserved without compromise.
Summary Table:
| Process | Key Use Cases | Materials Benefited |
|---|---|---|
| Bright Annealing | Maintains clean, mirror-like surface | Stainless steels, reactive alloys |
| Aging | Prevents contamination during long soak times | Titanium, nickel-based alloys |
| Brazing | Ensures strong, oxide-free joints | Various metals in joining applications |
| Sintering | Protects powders from oxidation for dense parts | Metal powders in manufacturing |
| Quenching | Provides controlled cooling without oxidation | High-value, reactive metals |
Need precise heat treatment solutions? KINTEK leverages exceptional R&D and in-house manufacturing to provide advanced high-temperature furnaces like Muffle, Tube, Rotary, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems. With strong deep customization capabilities, we tailor our solutions to meet your unique experimental needs, ensuring optimal performance for reactive metals and high-quality finishes. Contact us today to discuss how we can enhance your processes!
Visual Guide
Related Products
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
People Also Ask
- What is the main purpose of heat treatment? Transform Metal Properties for Superior Performance
- What is the use of nitrogen in furnace? Prevent Oxidation for Superior Heat Treatment
- What are the environmental benefits of using inert gases in furnaces? Reduce Waste and Emissions for a Greener Process
- How does nitrogen atmosphere heat treatment improve surface strengthening? Enhance Durability and Performance
- How does the inert atmosphere heat treating process work? Prevent Oxidation for Superior Material Quality



















