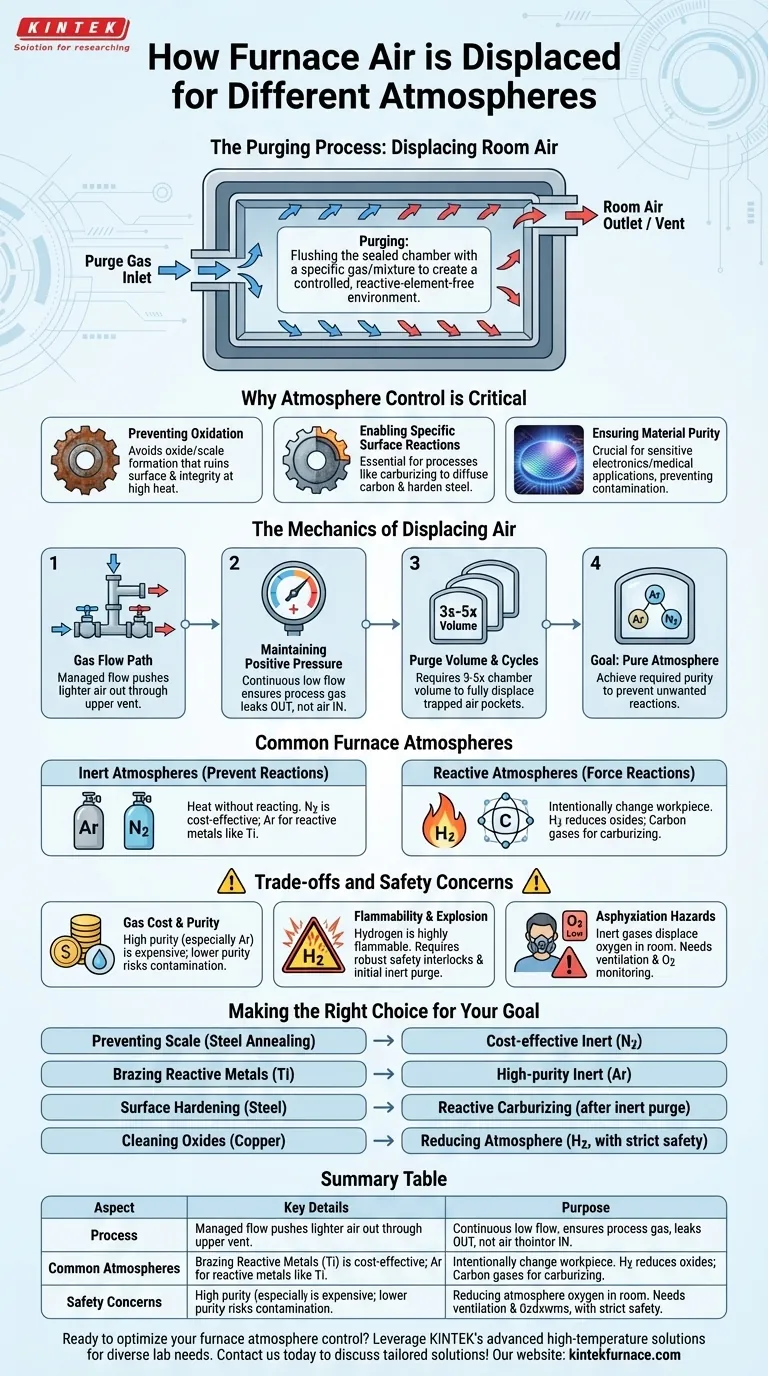
To operate in different atmospheres, a furnace systematically displaces the ambient room air through a process known as purging. This involves using dedicated gas inlets to flush the sealed heating chamber with a specific gas or gas mixture, pushing the original air out through an outlet or vent. The goal is to create a controlled environment that is free from the reactive elements in air, primarily oxygen and water vapor.
The core challenge is not simply filling a chamber with a new gas, but ensuring this new atmosphere is pure enough to prevent unwanted chemical reactions with the workpiece at high temperatures. Effective air displacement is a critical process control variable for achieving desired material properties.

Why Atmosphere Control is Critical
At room temperature, air is relatively benign. At the high temperatures found inside a furnace, however, the oxygen and moisture in the air become highly reactive, fundamentally altering the materials being processed.
Preventing Oxidation and Contamination
The most common reason to displace room air is to prevent oxidation. When heated, most metals will readily react with oxygen to form a layer of oxide (scale or rust) on their surface. This can ruin surface finish, alter dimensions, and compromise the material's structural integrity.
Enabling Specific Surface Reactions
Conversely, some processes require a specific, highly controlled reactive atmosphere. For example, in carburizing, a carbon-rich gas is introduced to diffuse carbon into the surface of steel, hardening it. Displacing the air is the first step before introducing this precise gas mixture.
Ensuring Material Purity
For sensitive materials used in electronics or medical applications, even trace amounts of contamination can be detrimental. Purging with a high-purity inert gas ensures that no unintended elements are introduced into the material during the heating cycle.
The Mechanics of Displacing Air
The process of purging is a deliberate engineering control designed to ensure a complete and safe gas exchange within the furnace chamber.
Gas Flow Path: Inlets and Outlets
Furnaces designed for atmospheric control have at least one gas inlet and one outlet. The purge gas is introduced through the inlet, and its flow is managed to systematically "push" the lighter air out of the chamber, typically through an upper vent or outlet.
Maintaining Positive Pressure
During operation, a continuous, low-volume flow of the desired gas is often maintained. This creates a slight positive pressure inside the furnace, ensuring that if any minor leaks exist in the door seals, the process gas will flow out rather than allowing room air to leak in.
Purge Volume and Cycles
A single, quick flush is rarely sufficient. A common rule of thumb is to purge the chamber with a volume of gas equal to three to five times the volume of the chamber itself. This ensures that pockets of trapped air are fully displaced and the atmosphere reaches the required purity level.
Common Furnace Atmospheres and Their Purpose
The choice of gas is determined entirely by the process goal. The gases mentioned in the references fall into two main categories.
Inert Atmospheres: Preventing All Reactions
Gases like Argon (Ar) and Nitrogen (N2) are chemically inert. They are used when the goal is simply to heat a material without it reacting with its surroundings. Nitrogen is a cost-effective choice for many applications, while argon is used for more reactive metals (like titanium) where nitrogen could form nitrides.
Reactive Atmospheres: Forcing a Reaction
Reactive atmospheres are used to intentionally change the workpiece. A hydrogen (H2) mixture, for example, is a reducing agent and can be used to remove oxides from a metal's surface. As mentioned earlier, carbon-based gases are used for carburizing, and controlled amounts of oxygen (O2) can be used for specific oxidation processes.
Understanding the Trade-offs and Safety Concerns
While essential, creating a controlled atmosphere introduces complexity, cost, and significant safety risks that must be managed.
Gas Cost and Purity
High-purity gases, especially argon, can be expensive. The cost of the gas and the volume required for effective purging can be a significant operational expense. Using a lower-purity gas to save money can compromise the entire process by introducing contaminants.
Flammability and Explosion Risk
Hydrogen is highly flammable and can be explosive when mixed with air. Furnaces using hydrogen must have robust safety interlocks. This includes performing an initial purge with an inert gas like nitrogen to remove all oxygen before the hydrogen is introduced.
Asphyxiation Hazards
Inert gases like nitrogen and argon are silent threats. They displace oxygen not only in the furnace but also in the surrounding room if a major leak occurs. This creates a serious asphyxiation hazard for personnel, requiring proper ventilation and oxygen monitoring in the workspace.
Making the Right Choice for Your Goal
Selecting the correct atmosphere is a function of your material and your desired outcome. Use the principles above to guide your decision-making.
- If your primary focus is preventing surface scale on steel during annealing: Use a cost-effective inert atmosphere like Nitrogen to displace the oxygen.
- If your primary focus is brazing reactive metals like titanium: Use a high-purity inert gas like Argon to prevent the formation of both oxides and nitrides.
- If your primary focus is surface hardening a steel component: Use a reactive carburizing atmosphere after first purging all air with an inert gas.
- If your primary focus is cleaning oxides from copper parts: Use a reducing atmosphere containing Hydrogen, ensuring all safety protocols for flammability are strictly followed.
Mastering atmospheric control transforms the furnace from a simple heater into a precision instrument for material engineering.
Summary Table:
| Aspect | Key Details |
|---|---|
| Purpose | Displace air to prevent oxidation, enable reactions, ensure material purity |
| Process | Purging with gas via inlets/outlets, maintaining positive pressure, 3-5 chamber volume cycles |
| Common Atmospheres | Inert (e.g., Nitrogen, Argon) for prevention; Reactive (e.g., Hydrogen, carbon gases) for specific reactions |
| Safety Concerns | Flammability risks (e.g., Hydrogen), asphyxiation hazards from inert gases, gas cost and purity trade-offs |
Ready to optimize your furnace atmosphere control? Leveraging exceptional R&D and in-house manufacturing, KINTEK provides diverse laboratories with advanced high-temperature furnace solutions. Our product line, including Muffle, Tube, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems, is complemented by our strong deep customization capability to precisely meet unique experimental requirements. Contact us today to discuss how our tailored solutions can enhance your material processing and safety!
Visual Guide
Related Products
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- What does inert mean in furnace atmospheres? Protect materials from oxidation with inert gases.
- What is the main purpose of heat treatment? Transform Metal Properties for Superior Performance
- How does the inert atmosphere heat treating process work? Prevent Oxidation for Superior Material Quality
- What is nitrogen used for in a furnace? Prevent Oxidation and Control Heat Treatment Quality
- What are the benefits of inert atmosphere heat treating? Prevent Oxidation and Preserve Material Integrity



















