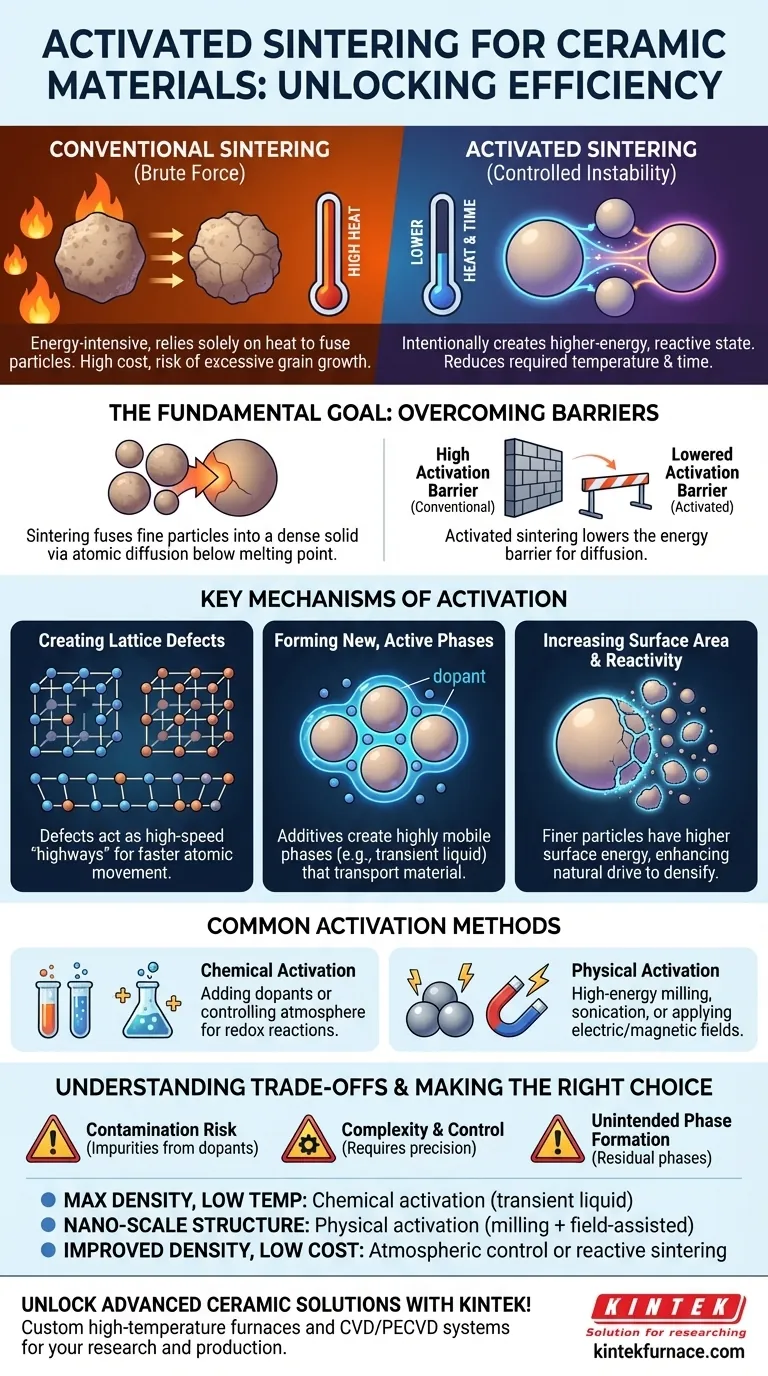
At its core, activated sintering is a group of advanced techniques used to make the densification of ceramic powders happen more easily and efficiently. It achieves this by intentionally putting the material into a higher-energy, more reactive state either before or during the heating process, which significantly reduces the required sintering temperature and time.
Conventional sintering relies solely on high heat to force particles together, an energy-intensive process. Activated sintering fundamentally changes this by introducing controlled instability—such as lattice defects or reactive chemical phases—that provides easier pathways for atoms to move, enabling densification with far less thermal energy.
The Fundamental Goal: Overcoming Sintering Barriers
What is Sintering?
Sintering is the process of taking a collection of fine particles, often compacted into a shape called a "green body," and heating them to a temperature below their melting point. This heat gives atoms enough energy to diffuse across the boundaries of the particles, fusing them together into a solid, dense object.
The Problem with Conventional Sintering
The primary challenge is that very high temperatures are needed to initiate this atomic diffusion. This high thermal energy is not only costly but can also lead to unwanted side effects, such as excessive grain growth, which can degrade the mechanical properties of the final ceramic part.
Activated Sintering's Solution
Activated sintering provides the necessary energy through means other than just heat. By creating a higher-energy state in the powder, you lower the activation barrier for diffusion. This means the process can be successfully completed at a lower temperature, for a shorter duration, or both.
Key Mechanisms of Activation
Creating Lattice Defects
One of the most effective activation methods is to introduce defects—such as vacancies or dislocations—into the crystal lattice of the powder particles. These defects act as high-speed "highways" for atoms to travel along, dramatically accelerating the rate of diffusion and, therefore, densification.
Forming New, Active Phases
Another powerful technique involves adding small amounts of a substance, or "dopant," to the primary powder. This additive can react at the particle surfaces to form a new, highly mobile phase at a temperature much lower than the sintering temperature of the main material. This could be a temporary liquid phase that "wets" the particles or a highly reactive solid solution at the grain boundaries, both of which serve to rapidly transport material and close pores.
Increasing Surface Area and Reactivity
The fundamental driving force for sintering is the reduction of surface energy. Finer particles have a much higher surface-area-to-volume ratio, making them inherently more energetic and unstable. Physical processing that reduces particle size, therefore, "activates" the powder by increasing its natural drive to densify.
Common Activation Methods
Chemical Activation
This involves altering the chemistry of the system. It can be achieved by adding dopants that form the active phases mentioned above, or by controlling the furnace atmosphere to trigger reduction-oxidation (redox) reactions at the particle surfaces, which creates a highly mobile, non-stoichiometric state.
Physical Activation
This category uses physical forces to energize the material. High-energy milling or sonication can be used before sintering to reduce particle size and introduce a high density of lattice defects. Applying an electric or magnetic field during sintering (as in Field-Assisted Sintering or Spark Plasma Sintering) can also directly accelerate diffusion pathways.
Understanding the Trade-offs
The Risk of Contamination
Chemical activation relies on additives. If not chosen carefully or if they do not fully diffuse away or become incorporated benignly, these dopants can remain as impurities in the final ceramic, potentially compromising its performance.
Complexity and Process Control
Activated sintering methods are inherently more complex than conventional "heat and hold" techniques. They require precise control over additive concentrations, atmospheric conditions, or applied fields. A loss of control can lead to non-uniform densification or undesirable microstructures.
Unintended Phase Formation
While the goal is often to create a temporary, beneficial phase, there is a risk that this phase becomes trapped or is not the one intended. Such residual phases at the grain boundaries can become points of mechanical weakness.
Making the Right Choice for Your Goal
Choosing an activation strategy depends entirely on the desired outcome for your material.
- If your primary focus is achieving maximum density at the lowest temperature: Chemical activation using a dopant designed to create a transient liquid phase is often the most effective approach.
- If your primary focus is preserving a nano-scale grain structure: Prioritize physical activation like high-energy milling combined with a rapid, field-assisted sintering technique to minimize the time spent at high temperatures.
- If your primary focus is improving densification with minimal cost: Consider atmospheric control or reactive sintering, which may provide significant benefits without requiring specialized additives or equipment.
Ultimately, activated sintering transforms the process from a brute-force application of heat into a precise, scientifically controlled method for engineering superior ceramic materials.

Summary Table:
| Activation Method | Key Mechanism | Benefits |
|---|---|---|
| Chemical Activation | Introduces dopants to form reactive phases | Lowers sintering temperature, improves densification |
| Physical Activation | Uses milling or fields to create defects | Accelerates diffusion, reduces grain growth |
| Surface Area Increase | Enhances particle reactivity via finer powders | Boosts densification drive, lowers energy needs |
Unlock advanced ceramic solutions with KINTEK! Leveraging exceptional R&D and in-house manufacturing, we provide diverse laboratories with high-temperature furnace solutions like Muffle, Tube, Rotary, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems. Our strong deep customization capability ensures precise alignment with your unique experimental needs, helping you achieve superior material properties efficiently. Contact us today to discuss how our tailored sintering technologies can elevate your research and production outcomes!
Visual Guide
Related Products
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- Multi Zone Laboratory Quartz Tube Furnace Tubular Furnace
People Also Ask
- What role does a muffle furnace play in the preparation of MgO support materials? Master Catalyst Activation
- What substances are prohibited from being introduced into the furnace chamber? Prevent Catastrophic Failure
- What environmental conditions are critical for SiOC ceramicization? Master Precise Oxidation & Thermal Control
- What is the key role of a muffle furnace in the pretreatment of boron sludge and szaibelyite? Unlock Higher Process Efficiency
- What is the role of a muffle furnace in the synthesis of water-soluble Sr3Al2O6? Precision in SAO Production



















