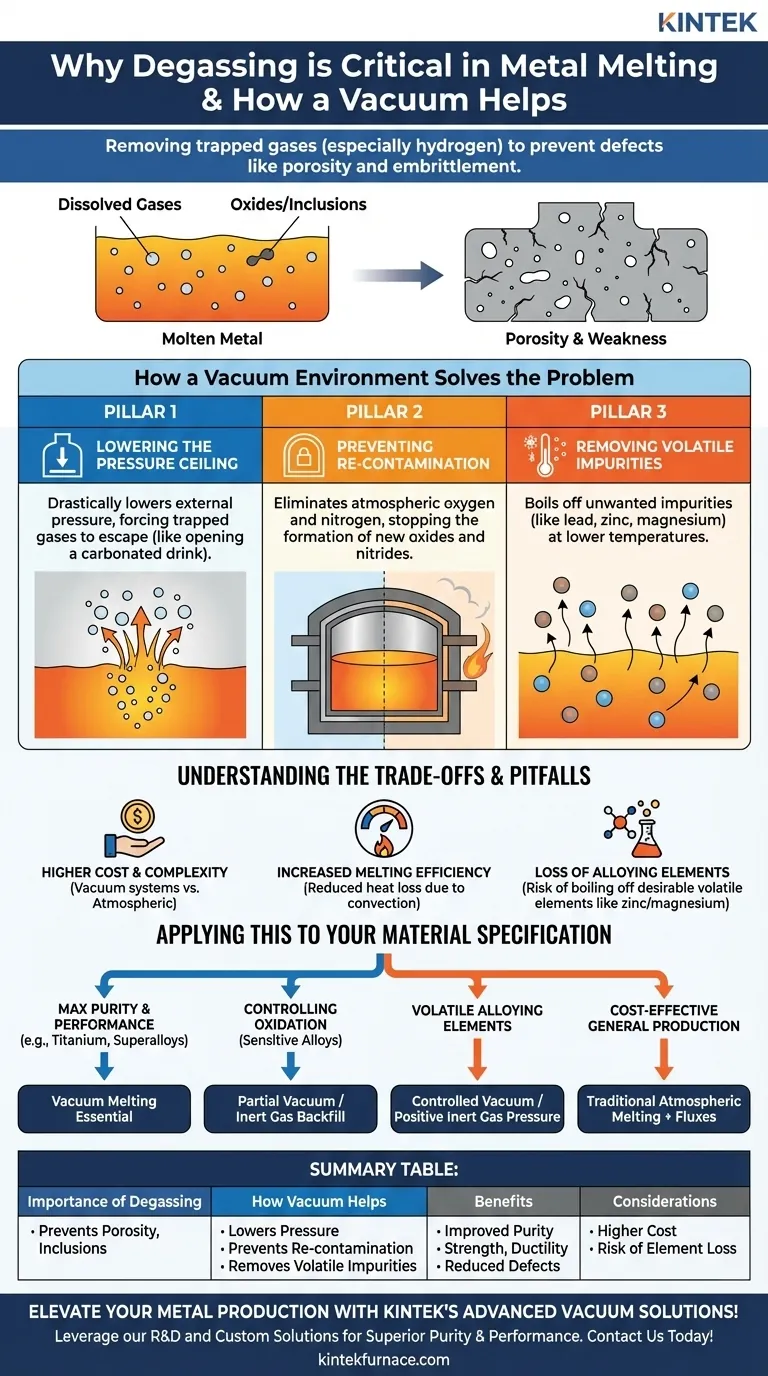
In metal production, degassing is a critical purification step used to remove dissolved gases, primarily hydrogen, from the molten metal. These trapped gases are a major source of defects like porosity, which compromises the structural integrity of the final product. A vacuum environment dramatically accelerates degassing by lowering the atmospheric pressure above the melt, creating a powerful force that pulls the unwanted gases out.
Uncontrolled gases and impurities trapped in molten metal are a primary cause of defects and inconsistent material properties. Using a vacuum doesn't just passively remove these gases; it fundamentally alters the physics of the melting environment to actively prevent contamination and purify the alloy, ensuring maximum integrity in the final casting.

The Fundamental Problem: Contamination in Molten Metal
To understand why degassing is essential, we must first recognize that molten metal acts as a solvent for gases and other impurities from its environment.
Gases Lead to Porosity and Embrittlement
Gases like hydrogen, oxygen, and nitrogen readily dissolve in metal when it is in its liquid state. As the metal cools and solidifies, its ability to hold these gases in solution drops sharply.
The gases are then forced out of solution, forming microscopic bubbles. These bubbles become trapped within the solidifying metal structure, creating voids known as porosity. This porosity acts as a network of internal cracks, severely reducing the material's strength, ductility, and fatigue life.
Oxides and Inclusions Degrade Performance
Reactive gases, especially oxygen from the air, combine with the molten metal to form non-metallic compounds called oxides.
These microscopic oxide particles, known as inclusions, do not blend into the metal's crystalline structure. Instead, they disrupt it, acting as stress concentration points where cracks can easily initiate under load, making the material brittle and prone to premature failure.
How a Vacuum Environment Solves the Problem
Creating a vacuum during the melting process addresses these issues directly by manipulating the physical and chemical environment.
Principle 1: Lowering the Pressure Ceiling
The amount of gas a liquid can hold is determined by the pressure of that gas above the liquid's surface. A vacuum drastically lowers this external pressure.
This creates a significant pressure differential between the dissolved gas inside the melt and the near-zero pressure outside of it. Like opening a carbonated drink, this imbalance provides a powerful driving force for the dissolved gases to escape the liquid metal.
Principle 2: Preventing Re-Contamination
Atmospheric air is composed of roughly 21% oxygen and 78% nitrogen, both of which are highly reactive with molten metals.
By evacuating the air from the melting chamber, a vacuum eliminates the source of contamination. This prevents the formation of new oxides and nitrides, ensuring the metal remains clean throughout the melting and pouring process.
Principle 3: Removing Volatile Impurities
A vacuum also enables a powerful purification process based on vapor pressure. Every element has a temperature and pressure at which it will "boil" or vaporize.
Under a vacuum, unwanted tramp elements with high vapor pressures (like lead, zinc, or magnesium) can be boiled out of the melt at a temperature far below the melting point of the primary metal. This allows for the selective removal of metallic impurities, achieving an even higher level of purity.
Understanding the Trade-offs and Pitfalls
While highly effective, vacuum processing is a specialized technique with specific considerations.
The Cost and Complexity of Vacuum Systems
Vacuum furnaces are significantly more complex and expensive to purchase, operate, and maintain than furnaces that operate at atmospheric pressure. This investment is the primary trade-off for achieving superior metal quality.
Increased Melting Efficiency
A secondary benefit of a vacuum is improved thermal efficiency. With no air in the chamber, heat loss due to convection is eliminated. Heat is transferred primarily through radiation, leading to faster and more uniform melting with potentially lower energy consumption.
A Critical Pitfall: Loss of Alloying Elements
The same principle that removes impurities can also work against you. If a desirable alloying element has a high vapor pressure, melting under a deep vacuum can cause it to boil off.
This is a critical concern for alloys containing elements like zinc (in brass) or magnesium (in some aluminum alloys). In these cases, the vacuum level must be precisely controlled, or a positive pressure of an inert gas like argon may be used to suppress this vaporization.
Applying This to Your Material Specification
Your choice of melting process should be directly tied to the performance requirements and cost constraints of your application.
- If your primary focus is maximum purity and performance: Vacuum melting is non-negotiable for reactive metals (like titanium), superalloys, and medical-grade materials where porosity and inclusions are unacceptable.
- If your primary focus is controlling oxidation in sensitive alloys: A partial vacuum or an inert gas backfill (like argon) can provide excellent protection from the atmosphere at a lower cost than a deep vacuum system.
- If your alloy contains volatile elements: You must carefully control the vacuum level or use a positive inert gas pressure to prevent the loss of critical alloying components.
- If your primary focus is cost-effective production of general-purpose metals: Traditional atmospheric melting combined with chemical deoxidizers and fluxes is often sufficient and the most economical choice.
Ultimately, understanding the role of a vacuum transforms it from a mere process step into a precise tool for engineering the fundamental properties of your material.
Summary Table:
| Aspect | Key Points |
|---|---|
| Importance of Degassing | Removes dissolved gases (e.g., hydrogen) to prevent porosity, embrittlement, and inclusions, ensuring structural integrity. |
| How Vacuum Helps | Lowers pressure to force gas removal, prevents re-contamination, and removes volatile impurities via vapor pressure. |
| Benefits | Improved metal purity, strength, ductility, and fatigue life; reduced defects in castings. |
| Considerations | Higher cost and complexity; risk of losing volatile alloying elements; suitable for reactive metals and high-performance alloys. |
Elevate Your Metal Production with KINTEK's Advanced Vacuum Solutions!
Leveraging exceptional R&D and in-house manufacturing, KINTEK provides diverse laboratories with advanced high-temperature furnace solutions, including Vacuum & Atmosphere Furnaces and CVD/PECVD Systems. Our strong deep customization capability ensures we precisely meet your unique experimental requirements, whether you're working with reactive metals, superalloys, or other materials. Achieve superior purity, performance, and efficiency in your metal melting processes—contact us today to discuss how our tailored solutions can benefit your operations!
Visual Guide
Related Products
- Vacuum Induction Melting Furnace
- 600T Vacuum Induction Hot Press Vacuum Heat Treat and Sintering Furnace
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
People Also Ask
- Why is a Vacuum Induction Melting (VIM) furnace essential? Unlock Purity for Aerospace and Semiconductors
- How does vacuum melting technology contribute to sustainability? Boost Durability and Recycling Efficiency
- What role does a vacuum induction melting furnace play in Fe-5%Mn-C alloys? Ensure Chemical Integrity and High Purity
- How has vacuum smelting impacted the development of superalloys? Unlock Higher Strength and Purity
- What are the common applications of Vacuum Induction Melting? Essential for High-Performance Metals and Alloys



















