At its core, refractory metals like tungsten are used in vacuum furnaces for their exceptional ability to withstand extreme heat without melting. However, these same metals are highly reactive with oxygen, and the vacuum environment is absolutely essential to protect them from rapidly burning up and failing at temperatures they are otherwise designed to endure.
The use of tungsten in a vacuum furnace is not a matter of choice, but a fundamental necessity. The vacuum shields the metal from oxygen, enabling its extraordinary heat resistance to be utilized, while the metal provides the high-temperature capability the furnace requires.
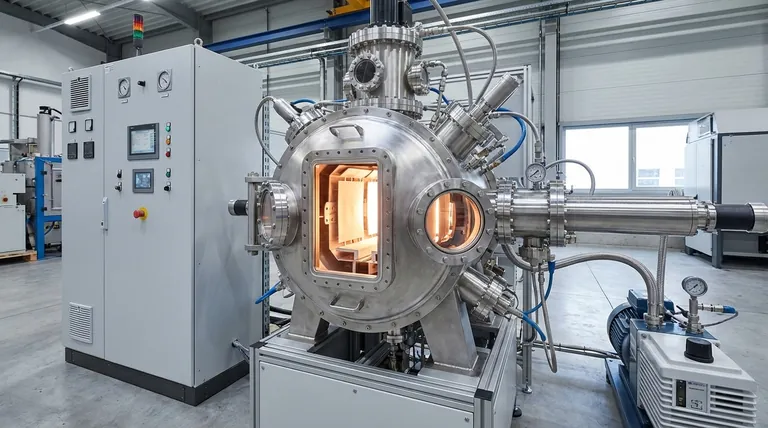
The Core Challenge: High Temperature Without Self-Destruction
To understand this pairing, you must see it as a solution to a fundamental engineering problem: how to generate immense heat without the heating system destroying itself.
Defining "Refractory": Extreme Heat Resistance
The term "refractory" describes materials with an exceptionally high melting point and thermal stability. Tungsten, with a melting point of 3422°C (6192°F), is a prime example.
This property allows it to serve as a heating element, glowing white-hot to radiate enormous amounts of energy without losing its structural integrity.
The Achilles' Heel: Rapid Oxidation
The critical weakness of refractory metals is their intense reactivity with oxygen. Even at moderately high temperatures, often as low as 300–500°C, tungsten will rapidly oxidize.
This oxidation process effectively burns the metal, causing it to become brittle, degrade, and ultimately fail catastrophically. This makes it completely unsuitable for use in a standard air-atmosphere furnace.
The Vacuum as a Protective Shield
A vacuum furnace solves this problem by removing the atmosphere, specifically the reactive oxygen, from the heating chamber.
By operating in a near-vacuum, the furnace creates an inert environment. This protective shield prevents oxidation, allowing the tungsten elements and shielding to operate effectively at temperatures exceeding 2000°C.
Understanding the Trade-offs
Choosing tungsten for a vacuum furnace involves accepting a clear set of trade-offs. It is a high-performance solution with specific vulnerabilities.
High Performance vs. High Cost
Refractory metals are inherently expensive to source and difficult to machine. The initial investment in tungsten heating elements and hot zone shielding is significant compared to alternatives like graphite.
This cost is justified only when the process demands temperatures or purity levels that other materials cannot achieve.
Environmental Sensitivity vs. Durability
Inside a properly maintained vacuum, tungsten components are incredibly durable and long-lasting. However, the entire system is sensitive to its environment.
A small air leak, improper purging, or contamination can introduce enough oxygen to cause rapid failure of the expensive refractory components. The integrity of the vacuum is paramount.
Tungsten vs. Other Materials
Molybdenum is another refractory metal used in furnaces, but it has a lower melting point than tungsten, making it suitable for a slightly lower temperature range.
Graphite is a common, cost-effective alternative for many applications but can be a source of carbon contamination, which is unacceptable for certain sensitive materials and alloys.
Making the Right Choice for Your Process
The decision to use tungsten is dictated entirely by your specific processing requirements and operational goals.
- If your primary focus is reaching the highest possible temperatures (>2000°C): Tungsten is the definitive choice due to its unparalleled melting point and stability in a vacuum.
- If your primary focus is processing highly sensitive, non-carbon-reactive materials: A refractory metal hot zone is superior to graphite for maintaining a clean, pure environment.
- If your primary focus is cost-effectiveness for mid-range temperatures (<2000°C): Molybdenum or high-purity graphite often provide a more economical solution without sacrificing performance.
Ultimately, using tungsten in a vacuum furnace is a calculated decision to trade environmental sensitivity for unmatched high-temperature performance.
Summary Table:
| Property | Tungsten in Vacuum Furnaces |
|---|---|
| Melting Point | 3422°C (6192°F) |
| Key Advantage | Extreme heat resistance without melting |
| Critical Weakness | Rapid oxidation in oxygen |
| Solution | Vacuum environment prevents oxidation |
| Common Applications | High-temperature processing (>2000°C), sensitive materials |
| Trade-offs | High cost, environmental sensitivity |
Need a reliable high-temperature furnace solution? Leveraging exceptional R&D and in-house manufacturing, KINTEK provides diverse laboratories with advanced solutions like Muffle, Tube, Rotary, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems. Our strong deep customization capability ensures we precisely meet your unique experimental requirements. Contact us today to enhance your lab's efficiency and achieve superior results!
Visual Guide
Related Products
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Laboratory Muffle Oven Furnace with Bottom Lifting
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Multi Zone Laboratory Quartz Tube Furnace Tubular Furnace
People Also Ask
- Why are vacuum furnaces important for stainless steel processing? Ensure Corrosion Resistance and Pristine Finishes
- What are the advantages of graphite's lightweight and high strength in vacuum furnaces? Lower Costs & Superior Performance
- How are vacuum furnaces classified based on chamber design? Explore Horizontal, Vertical, and Bottom-Loading Types
- What is the purpose of using an industrial vacuum resistance furnace? Enhancing Ti-33Mo-0.2C Alloy Performance
- What is the importance of a gas pressure sintering furnace for Silicon Nitride? Achieve High-Performance Densification
- What is the function of the crystallizer in a vacuum sublimation furnace? Mastering Temperature for High-Purity Magnesium
- How do vacuum furnaces contribute to the production of advanced ceramic materials? Achieve Superior Purity and Density
- What is the purpose of transferring high-temperature glass to a preheated annealing furnace? Ensuring Sample Integrity



















