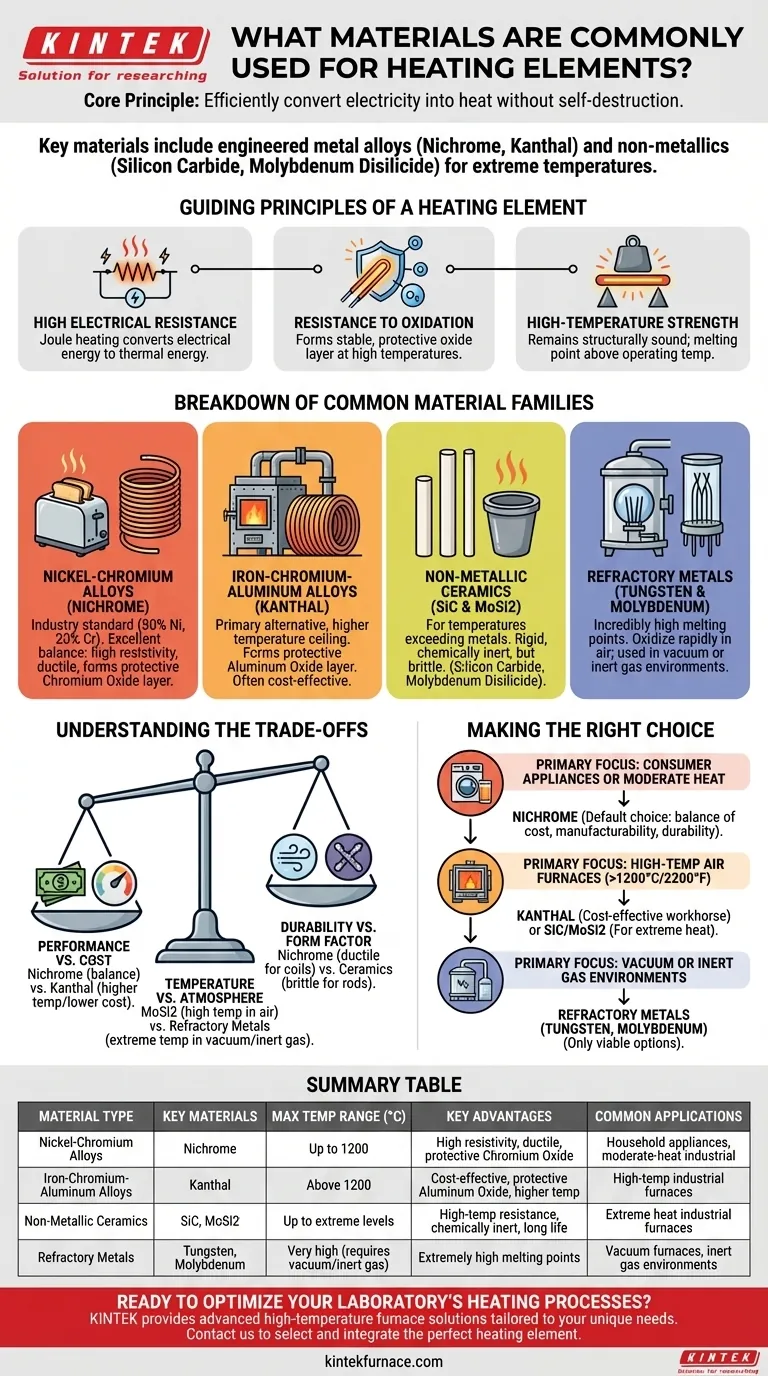
At its core, a heating element's material is chosen based on its ability to efficiently convert electricity into heat without destroying itself. The most common materials are metal alloys specifically engineered for this task, primarily Nichrome (a nickel-chromium alloy) and Kanthal (an iron-chromium-aluminum alloy). For more extreme temperatures, non-metallic materials like Silicon Carbide and Molybdenum Disilicide are used.
The selection of a heating element material is not about finding the one with the highest resistance. It is a calculated trade-off between electrical resistivity, resistance to high-temperature oxidation, and mechanical durability within a specific operating environment.

The Guiding Principles of a Heating Element
To understand why certain materials are used, we must first understand the fundamental challenges a heating element must overcome. The ideal material must excel in three key areas.
High Electrical Resistance
A heating element works by passing an electrical current through a material that resists the flow of electrons. This resistance converts electrical energy into thermal energy, a phenomenon known as Joule heating. Materials with higher resistivity generate more heat for a given current.
Resistance to Oxidation
This is the most critical factor for an element's lifespan. At high temperatures, most metals rapidly react with oxygen in the air, causing them to corrode and fail. The best heating element materials, like Nichrome, form a stable, protective oxide layer on their surface that prevents further oxidation, even when glowing red-hot.
High-Temperature Strength
The material must have a melting point significantly higher than its operating temperature. It also needs to remain structurally sound and not become overly brittle or soft when heated repeatedly.
A Breakdown of Common Material Families
Heating element materials can be grouped into distinct families, each suited for different applications and temperature ranges.
Nickel-Chromium Alloys (Nichrome)
Nichrome (typically 80% nickel, 20% chromium) is the industry standard for a vast range of applications, from household toasters to industrial heating processes.
Its popularity comes from an excellent balance of properties. It has high resistivity, is very ductile (easy to form into coils), and forms a durable, adherent layer of chromium oxide that protects it from degradation.
Iron-Chromium-Aluminum Alloys (Kanthal)
This family of alloys is the primary alternative to Nichrome, often used in high-temperature industrial furnaces. Kanthal can typically operate at higher temperatures than Nichrome.
These alloys form a protective layer of aluminum oxide, which has a higher melting point than chromium oxide, enabling superior performance at extreme heat. They are also generally less expensive than nickel-based alloys.
Non-Metallic Ceramics (SiC & MoSi2)
For temperatures that exceed the limits of even the best metal alloys, ceramic materials are used.
- Silicon Carbide (SiC) is a rigid, chemically inert material often formed into rods or tubes.
- Molybdenum Disilicide (MoSi2) can operate at exceptionally high temperatures and is known for its long service life in demanding industrial furnaces.
These materials are much more brittle than metal alloys and require careful design and support.
Refractory Metals (Tungsten & Molybdenum)
Metals like Tungsten and Molybdenum have incredibly high melting points. However, they oxidize almost instantly in the presence of air at high temperatures.
Because of this limitation, their use is restricted to specialized applications where oxygen is absent, such as in vacuum furnaces or environments filled with an inert gas.
Understanding the Trade-offs
Choosing a material is always an exercise in balancing competing factors. There is no single "best" material for all situations.
Performance vs. Cost
Nichrome offers excellent all-around performance and ductility, making it easy to manufacture into complex shapes for appliances. Kanthal offers a higher temperature ceiling and a lower material cost, making it a frequent choice for high-power industrial furnaces where raw performance is key.
Temperature vs. Atmosphere
While Molybdenum Disilicide can reach the highest temperatures in air, it is brittle. Refractory metals like Tungsten can go even hotter but are useless without a vacuum or inert gas to protect them from oxidation. The operating environment is as important as the temperature itself.
Durability vs. Form Factor
The ductility of metal alloys like Nichrome allows them to be easily wound into the tight coils needed for compact appliances like hair dryers. Brittle ceramics like Silicon Carbide cannot be formed this way and are typically used as rigid rods, which influences the design of the equipment they are used in.
Making the Right Choice for Your Application
Your selection should be guided by your primary operational goal.
- If your primary focus is consumer appliances or moderate-heat industrial processes: Nichrome is the default choice for its excellent balance of cost, manufacturability, and durability.
- If your primary focus is high-temperature air furnaces (above 1200°C / 2200°F): Kanthal (Fe-Cr-Al) alloys are a cost-effective workhorse, while Silicon Carbide and Molybdenum Disilicide are required for the most extreme heat.
- If your primary focus is specialized vacuum or inert gas environments: Refractory metals like Tungsten and Molybdenum are the only viable options due to their high melting points and susceptibility to oxidation.
Ultimately, selecting the right heating element material is a direct function of the operating temperature, environment, and required lifespan of the component.
Summary Table:
| Material Type | Key Materials | Max Temperature Range (°C) | Key Advantages | Common Applications |
|---|---|---|---|---|
| Nickel-Chromium Alloys | Nichrome | Up to 1200 | High resistivity, ductile, forms protective chromium oxide layer | Household appliances, moderate-heat industrial processes |
| Iron-Chromium-Aluminum Alloys | Kanthal | Above 1200 | Cost-effective, forms protective aluminum oxide layer, higher temperature capability | High-temperature industrial furnaces |
| Non-Metallic Ceramics | Silicon Carbide, Molybdenum Disilicide | Up to extreme levels | High-temperature resistance, chemically inert, long service life | Extreme heat industrial furnaces |
| Refractory Metals | Tungsten, Molybdenum | Very high (requires vacuum/inert gas) | Extremely high melting points | Vacuum furnaces, inert gas environments |
Ready to optimize your laboratory's heating processes? At KINTEK, we leverage exceptional R&D and in-house manufacturing to provide advanced high-temperature furnace solutions tailored to your unique needs. Our product line includes Muffle, Tube, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems, all supported by strong deep customization capabilities. Whether you're working with Nichrome, Kanthal, or specialized ceramics, we can help you select and integrate the perfect heating element for enhanced efficiency and durability. Contact us today to discuss how our solutions can benefit your specific applications and drive your research forward!
Visual Guide
Related Products
- Molybdenum Vacuum Heat Treat Furnace
- Silicon Carbide SiC Thermal Heating Elements for Electric Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
People Also Ask
- Why is a high vacuum essential for Ti-6Al-4V sintering? Protect Your Alloys from Embrittlement
- What is the purpose of a 1400°C heat treatment for porous tungsten? Essential Steps for Structural Reinforcement
- How does the ultra-low oxygen environment of vacuum sintering affect titanium composites? Unlock Advanced Phase Control
- What is the purpose of setting a mid-temperature dwell stage? Eliminate Defects in Vacuum Sintering
- Why is a high-vacuum environment necessary for sintering Cu/Ti3SiC2/C/MWCNTs composites? Achieve Material Purity



















