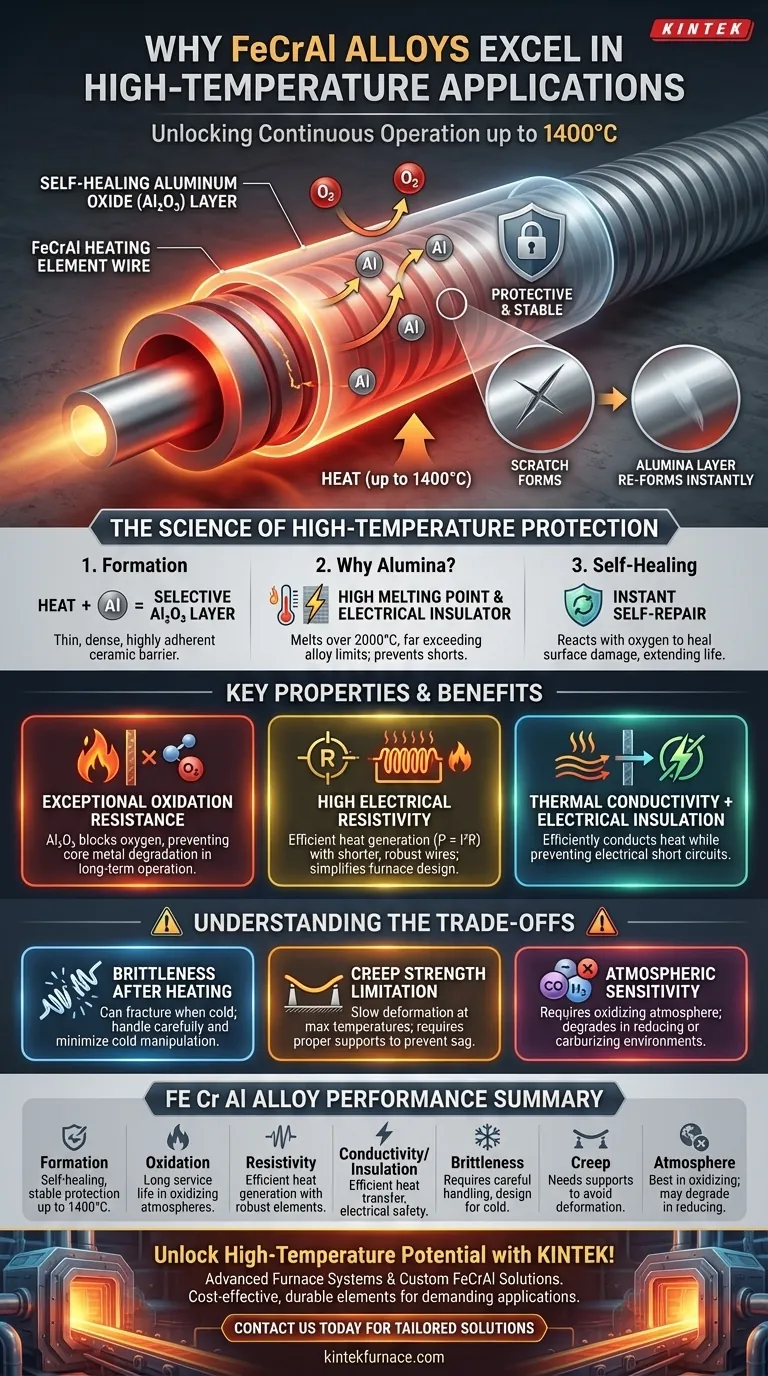
At the core of their high-temperature capability, Iron-Chrome-Aluminium (FeCrAl) alloys are engineered to form a stable, self-healing, and electrically insulating layer of aluminum oxide (Al₂O₃) on their surface when heated. This protective ceramic layer is the primary reason they can operate continuously at temperatures up to 1400°C, providing exceptional oxidation resistance and long service life in demanding environments like industrial furnaces.
FeCrAl alloys are not just resistant to heat; they are designed to create their own protective ceramic coating in situ. This unique aluminum oxide layer provides both exceptional oxidation resistance and high electrical resistivity, a combination that makes them a superior and cost-effective choice for electric heating elements.

The Science of High-Temperature Protection
The performance of FeCrAl alloys stems from a specific chemical reaction that occurs at high temperatures. Understanding this mechanism is key to understanding the material's value.
The Formation of the Alumina Layer
When a FeCrAl element is first heated, the aluminum (typically 4-7.5% of the composition) selectively migrates to the surface. There, it reacts with oxygen in the atmosphere to form a thin, dense, and highly adherent layer of aluminum oxide, also known as alumina (Al₂O₃).
Why Aluminum Oxide is the Key
This alumina layer is chemically inert and has a very high melting point (over 2000°C), far exceeding the operating temperature of the alloy itself (~1400°C) and its melting point (~1500°C). Unlike iron or chromium oxides, alumina is also an excellent electrical insulator.
Self-Healing Properties
This protective layer is not just a one-time formation. If the surface is scratched or damaged during operation, the exposed, hot alloy will immediately react with oxygen again, effectively "healing" the protective alumina barrier. This dramatically extends the component's service life.
Key Properties for Industrial Applications
The formation of the alumina layer gives FeCrAl alloys a unique combination of properties that make them ideal for electric heating.
Exceptional Oxidation Resistance
The dense Al₂O₃ layer acts as a barrier, preventing oxygen from reaching and degrading the underlying iron-chrome base metal. This allows for stable, long-term operation in oxidizing atmospheres where other metals would quickly fail.
High Electrical Resistivity
FeCrAl alloys possess a high electrical resistivity (around 145 μΩ-cm). For a heating element, this is a critical advantage. It allows the element to generate significant heat (P = I²R) from electrical current using a shorter and more robust wire, simplifying furnace design.
Thermal Conductivity with Electrical Insulation
This is the alloy's most powerful combination. The metallic core efficiently conducts heat to the furnace chamber, while the integral ceramic surface layer prevents electrical short circuits to support structures or the furnace shell.
Understanding the Trade-offs
No material is perfect. To use FeCrAl alloys effectively, you must be aware of their limitations.
Brittleness After Heating
After being subjected to high temperatures, FeCrAl alloys can become brittle once they cool to room temperature. This can make them difficult to service, reposition, or handle without fracturing. Design must account for this by minimizing the need for cold manipulation.
Creep Strength at Maximum Temperatures
Like all metals near their operating limits, FeCrAl can experience "creep"—a slow deformation under its own weight at high temperatures. Heating elements may sag over time and require proper ceramic supports to prevent distortion and failure.
Atmospheric Sensitivity
The protective mechanism of FeCrAl is dependent on an oxidizing atmosphere to form and maintain the Al₂O₃ layer. Its performance can be compromised in certain reducing or carburizing atmospheres that can attack and degrade the protective oxide.
Making the Right Choice for Your Application
Selecting the right material requires matching its properties to your primary operational goal.
- If your primary focus is cost-effective, long-life electric heating in an air-filled furnace: FeCrAl is almost always the superior choice due to its self-protecting nature and high resistivity.
- If your application involves significant vibration or requires frequent repositioning: You must design robust supports and plan maintenance procedures that account for the alloy's brittleness when cold.
- If you are operating in a specific non-oxidizing or contaminated atmosphere: You must verify the alloy's compatibility, as its protective mechanism relies on the presence of oxygen to function.
By understanding the mechanism of its protective oxide layer, you can effectively leverage the unique advantages of FeCrAl for reliable and efficient high-temperature performance.
Summary Table:
| Property | Benefit |
|---|---|
| Formation of Alumina Layer | Provides self-healing, stable protection up to 1400°C |
| High Oxidation Resistance | Ensures long service life in oxidizing atmospheres |
| High Electrical Resistivity | Enables efficient heat generation with shorter, robust elements |
| Thermal Conductivity with Electrical Insulation | Combines efficient heat transfer and electrical safety |
| Brittleness After Heating | Requires careful handling and design to prevent fractures |
| Creep Strength Limitation | Needs proper supports to avoid deformation at high temperatures |
| Atmospheric Sensitivity | Best suited for oxidizing environments; may degrade in reducing atmospheres |
Unlock the full potential of high-temperature solutions with KINTEK! Leveraging exceptional R&D and in-house manufacturing, we provide advanced furnace systems like Muffle, Tube, Rotary, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems. Our deep customization capabilities ensure precise alignment with your unique experimental needs, delivering cost-effective, durable heating elements for demanding applications. Contact us today to discuss how our tailored FeCrAl alloy solutions can enhance your lab's efficiency and reliability!
Visual Guide
Related Products
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- Split Multi Heating Zone Rotary Tube Furnace Rotating Tube Furnace
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
People Also Ask
- What is the primary function of a muffle furnace for BaTiO3? Master High-Temp Calcination for Ceramic Synthesis
- What is the role of a muffle furnace in the synthesis of water-soluble Sr3Al2O6? Precision in SAO Production
- How does a laboratory muffle furnace facilitate the biomass carbonization process? Achieve Precise Biochar Production
- What substances are prohibited from being introduced into the furnace chamber? Prevent Catastrophic Failure
- What environmental conditions are critical for SiOC ceramicization? Master Precise Oxidation & Thermal Control



















