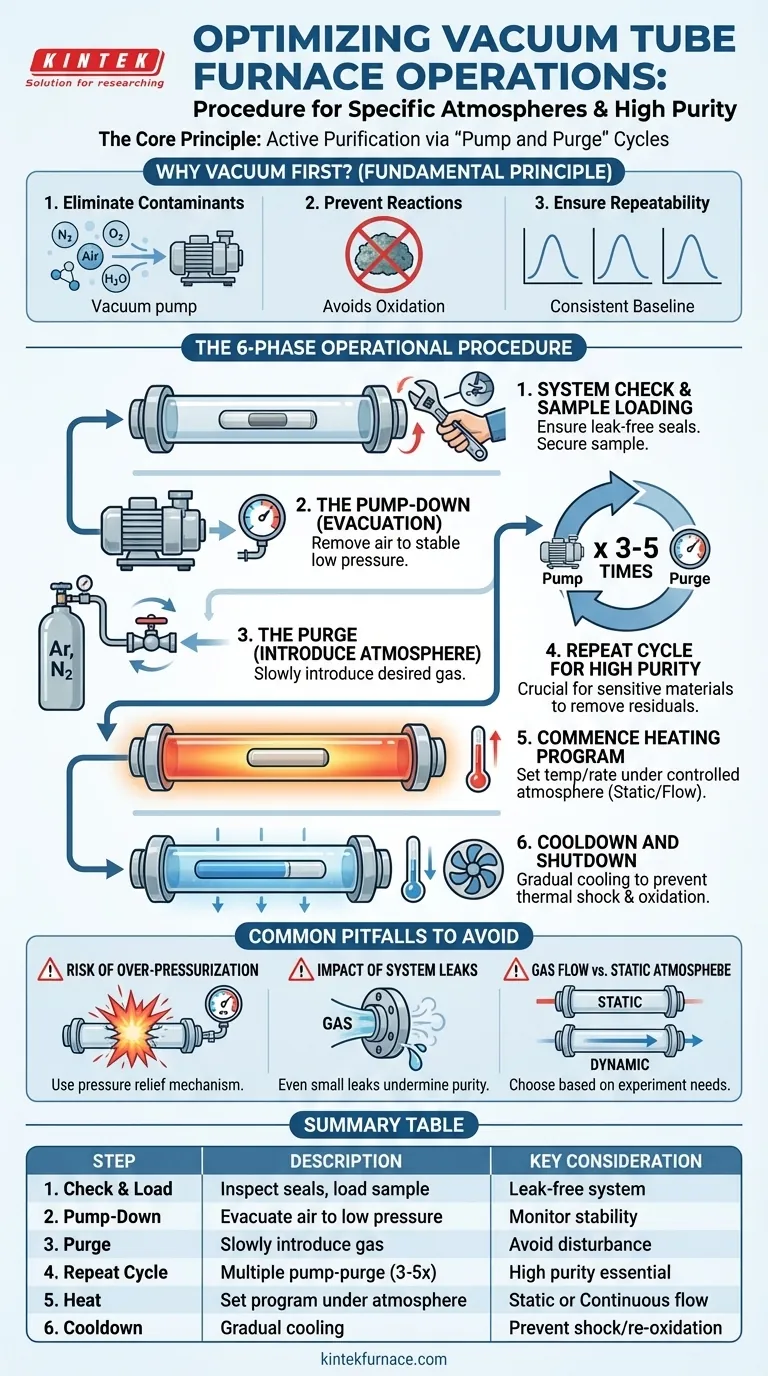
The recommended procedure for using a vacuum tube furnace with a specific atmosphere is to first evacuate the furnace tube using a vacuum pump. Once a sufficient vacuum is achieved, you then slowly introduce the desired atmosphere. For applications requiring high purity, this process of evacuating and backfilling with the target gas should be repeated multiple times to systematically remove residual atmospheric contaminants.
The core principle is not merely to replace the air, but to actively purify the internal environment. A repeated "pump and purge" cycle is the most effective method for minimizing contaminants like oxygen and moisture, ensuring the integrity and repeatability of your experiment.

The Fundamental Principle: Why Vacuum First?
Operating a tube furnace under a controlled atmosphere requires removing the ambient air that fills the tube at the start. Simply flowing your target gas into the tube is inefficient and often insufficient for achieving a pure environment.
Eliminating Atmospheric Contaminants
Ambient air is primarily nitrogen (~78%) and oxygen (~21%), with trace amounts of argon, carbon dioxide, and water vapor. Oxygen and water vapor are highly reactive at elevated temperatures and are often the primary contaminants you need to remove.
Preventing Unwanted Chemical Reactions
For many materials science and chemistry applications, the presence of oxygen can lead to unwanted oxidation, fundamentally altering your sample and invalidating your results. Evacuating the chamber first removes the vast majority of these reactive molecules.
Ensuring Experimental Repeatability
By starting with a vacuum and then introducing a known gas, you create a consistent and repeatable baseline for every experiment. This procedural discipline is critical for comparing results across different runs and ensuring your findings are reliable.
The Step-by-Step Operational Procedure
Following a strict sequence of operations is key to safety and success. This procedure can be broken down into six distinct phases.
Phase 1: System Check and Sample Loading
Before beginning, ensure all seals and connections on the furnace tube flanges are clean and properly fitted. Place your sample inside the tube, typically in the center of the heating zone, and then securely seal the tube. A leak-free system is non-negotiable.
Phase 2: The Pump-Down (Evacuation)
Connect the vacuum pump to the furnace tube's gas outlet port. Close the gas inlet and open the valve to the pump. Allow the pump to evacuate the air from the tube. Monitor the pressure using a vacuum gauge until it reaches a stable, low level.
Phase 3: The Purge (Introducing Atmosphere)
Once the target vacuum is reached, close the valve to the vacuum pump. Now, slowly open the gas inlet valve to introduce your desired atmosphere (e.g., Argon, Nitrogen). Do not open the valve quickly, as this can disturb your sample and create turbulence.
Phase 4: Repeating the Cycle for High Purity
For standard applications, one pump-and-purge cycle may be enough. However, for sensitive materials, it is best practice to repeat Phases 2 and 3. After the first purge, close the gas inlet, re-open the vacuum valve to pump out the gas mixture, and then purge again. Repeating this 3-5 times significantly increases the purity of the final atmosphere.
Phase 5: Commencing the Heating Program
With the final, pure atmosphere inside the tube, you can now begin the heating process. Set your target temperature and heating rates on the furnace controller. During the heating cycle, you can either maintain a static, sealed atmosphere or establish a slow, continuous flow of gas through the tube to carry away any outgassed products.
Phase 6: Cooldown and Shutdown
After the experiment is complete, the furnace heating elements are turned off. It is crucial to allow the furnace to cool down gradually, often under the same controlled atmosphere, to prevent thermal shock to the sample or furnace tube and to avoid re-introducing oxygen while the sample is still hot.
Understanding the Common Pitfalls
Proper procedure is as much about avoiding mistakes as it is about following steps. Awareness of these issues is critical for safe and effective operation.
Risk of Over-Pressurization
Never heat a completely sealed tube without a pressure relief mechanism. As the gas inside heats up, its pressure will increase significantly (in accordance with the ideal gas law). Always use an oil bubbler or a pressure relief valve on the outlet to prevent dangerous pressure buildup that could shatter the furnace tube.
The Impact of System Leaks
Even a small leak in a flange seal will continuously introduce atmospheric contaminants into your tube. This completely undermines the purpose of the pump-and-purge cycle. If you cannot achieve a good vacuum, check all seals before proceeding.
Gas Flow vs. Static Atmosphere
Decide whether your experiment needs a static (sealed) atmosphere or a dynamic (continuous flow) one. A continuous flow is better for removing byproducts from a reaction, but a static atmosphere may be required for processes sensitive to gas currents.
Applying This to Your Experiment
Your specific procedure should be tailored to the sensitivity of your materials and your experimental goals.
- If your primary focus is a standard annealing process: A single, thorough pump-down followed by introducing the atmosphere is often sufficient.
- If you are working with highly oxygen-sensitive materials: Perform a minimum of 3-5 pump-and-purge cycles to achieve the necessary high-purity environment.
- If your process generates gaseous byproducts: Use a continuous, low-flow rate of your chosen atmosphere during heating to sweep contaminants out of the reaction zone.
Mastering this procedure transforms the furnace from a simple heater into a precision instrument for controlling a material's chemical environment.
Summary Table:
| Step | Description | Key Consideration |
|---|---|---|
| 1. System Check & Sample Loading | Inspect seals, load sample, and seal tube | Ensure leak-free system for safety and purity |
| 2. Pump-Down (Evacuation) | Use vacuum pump to remove air from tube | Monitor pressure until stable low level is reached |
| 3. Purge (Introduce Atmosphere) | Slowly introduce desired gas (e.g., Argon) | Avoid rapid valve opening to prevent sample disturbance |
| 4. Repeat Cycle for High Purity | Perform multiple pump-and-purge cycles (3-5 times) | Essential for oxygen-sensitive materials to remove contaminants |
| 5. Commence Heating Program | Set temperature and heating rates under controlled atmosphere | Choose static or continuous flow based on experimental needs |
| 6. Cooldown and Shutdown | Cool furnace gradually under atmosphere | Prevent thermal shock and re-introduction of oxygen |
Ready to elevate your laboratory experiments with precise atmosphere control? KINTEK specializes in advanced high-temperature furnace solutions, including Vacuum Tube Furnaces, designed for diverse labs. With our strong R&D and in-house manufacturing, we offer deep customization to meet your unique requirements—ensuring purity, repeatability, and safety in your processes. Contact us today to discuss how our expertise can optimize your experimental outcomes!
Visual Guide
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- Multi Zone Laboratory Quartz Tube Furnace Tubular Furnace
People Also Ask
- What safety and reliability features are incorporated into a vertical tube furnace? Ensuring Safe, Consistent High-Temp Processing
- How is a Vertical Tube Furnace used for fuel dust ignition studies? Model Industrial Combustion with Precision
- What role does a laboratory tube furnace perform during the carbonization of LCNSs? Achieve 83.8% Efficiency
- How is a high-temperature tube furnace utilized in the synthesis of MoO2/MWCNTs nanocomposites? Precision Guide
- What recent improvements have been made to lab tube furnaces? Unlock Precision, Automation & Safety



















