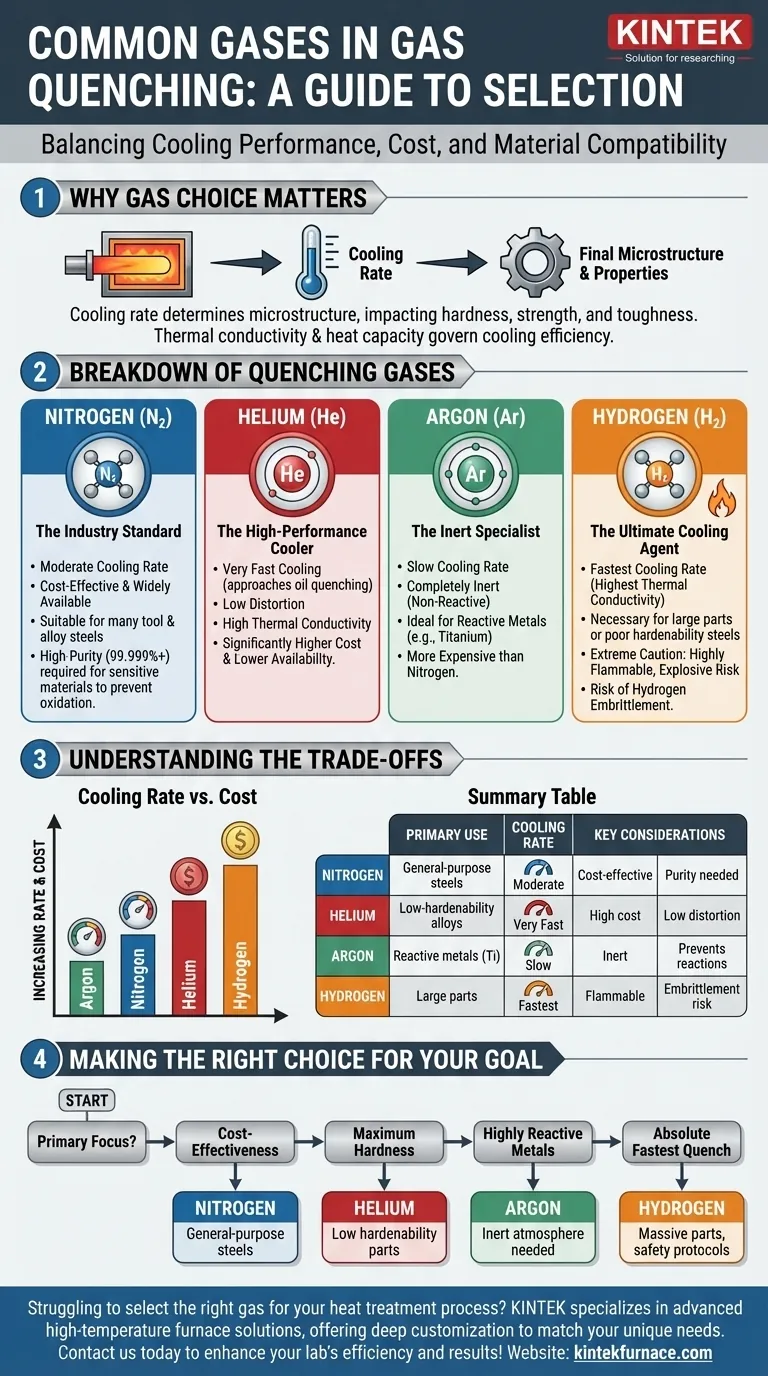
In modern heat treatment, the most common gases used for gas quenching are nitrogen, helium, argon, and hydrogen. The selection of a specific gas is not arbitrary; it is a critical engineering decision driven by the required cooling rate, the type of metal being treated, operational cost, and safety considerations.
The choice of a quenching gas represents a fundamental trade-off between cooling performance and cost. While nitrogen serves as the cost-effective industry standard, helium and hydrogen offer significantly faster cooling for demanding applications, and argon provides superior inertness for reactive metals.

Why the Choice of Gas Matters
In gas quenching, the gas is the medium responsible for extracting heat from the hot metal part at a specific, controlled rate. This cooling rate determines the final microstructure of the material, which in turn dictates its mechanical properties like hardness, strength, and toughness.
The Physics of Cooling
The cooling efficiency of a gas is primarily governed by its thermal conductivity and heat capacity. A gas with high thermal conductivity can transfer heat away from the part's surface much faster. This is why different gases produce vastly different results.
The Goal: Controlled Transformation
The objective is to cool the metal quickly enough to achieve the desired metallurgical phase—often martensite for steels—without causing excessive thermal stress, distortion, or cracking that can occur with harsh liquid quenches like water or oil.
A Breakdown of Common Quenching Gases
Each gas offers a unique profile of performance, cost, and material compatibility.
Nitrogen (N₂) — The Industry Standard
Nitrogen is the most widely used quenching gas due to its excellent balance of cost and performance. It is readily available, relatively inexpensive, and provides a moderate cooling rate suitable for many common tool steels and alloy steels.
For sensitive materials like high-alloy die steels or stainless steels, high-purity nitrogen (99.999% or higher) is used to prevent any surface oxidation or unwanted chemical reactions during the quench.
Helium (He) — The High-Performance Cooler
Helium has a much higher thermal conductivity than nitrogen. This allows it to achieve cooling rates that can approach those of oil quenching, but without the associated part distortion, cleaning costs, or environmental concerns.
Its primary disadvantage is its significantly higher cost and lower availability compared to nitrogen, reserving it for applications where maximum hardness or cooling speed is critical for low-hardenability alloys.
Argon (Ar) — The Inert Specialist
Argon's primary advantage is its complete inertness. It is even less reactive than nitrogen. This makes it the gas of choice for quenching highly reactive metals, such as titanium alloys, where even the slight risk of forming nitrides (a reaction possible with nitrogen) is unacceptable.
However, argon's thermal conductivity is lower than nitrogen's, resulting in a slower quench. It is also more expensive than nitrogen, limiting its use to these specialized cases.
Hydrogen (H₂) — The Ultimate Cooling Agent
Hydrogen possesses the highest thermal conductivity of any gas, delivering the fastest possible quench rates. This extreme cooling capability is necessary for very large cross-sections or steels with very poor hardenability.
Using hydrogen requires extreme caution. It is highly flammable and can form explosive mixtures with air. Furthermore, it can cause hydrogen embrittlement in certain steels, a phenomenon where the metal becomes brittle and fails unexpectedly. Its use is restricted to specialized furnaces and applications where the risks can be meticulously managed.
Understanding the Trade-offs
Selecting a quenching gas is an exercise in balancing competing priorities. There is no single "best" gas, only the most appropriate gas for a specific goal.
Cooling Rate vs. Cost
The relationship is direct and clear. For the fastest cooling, you must accept higher costs and complexity.
- Fastest: Hydrogen (highest cost & complexity)
- Very Fast: Helium (very high cost)
- Moderate: Nitrogen (cost-effective)
- Slowest: Argon (more expensive than nitrogen)
Gas Purity and Part Quality
Impurities like oxygen or moisture in the quench gas can cause undesirable surface defects like oxidation and decarburization. Using a high-purity gas, especially with high-value parts, ensures a clean, bright finish and preserves the integrity of the material's surface.
Reactivity and Material Compatibility
You must match the gas to the metal. While nitrogen is largely inert, it can react with elements like titanium, aluminum, and chromium at high temperatures to form nitrides. Hydrogen's risk of causing embrittlement makes it unsuitable for many ferrous alloys. Argon is the solution for the most reactive materials.
Making the Right Choice for Your Goal
Base your decision on your primary technical and economic drivers.
- If your primary focus is cost-effectiveness for general-purpose tool and alloy steels: Nitrogen is the clear and logical default choice.
- If your primary focus is achieving maximum hardness in parts with low hardenability: Helium provides a high-performance quench without the safety risks of hydrogen.
- If your primary focus is quenching highly reactive metals like titanium: Argon is the only choice to guarantee an inert atmosphere and prevent unwanted surface reactions.
- If your primary focus is achieving the absolute fastest quench rate for massive parts: Hydrogen is the most powerful option, provided you have the specialized equipment and safety protocols to manage it.
By understanding these fundamental trade-offs, you can select the gas that precisely matches your metallurgical goals, operational constraints, and budget.
Summary Table:
| Gas | Primary Use | Cooling Rate | Key Considerations |
|---|---|---|---|
| Nitrogen | General-purpose tool and alloy steels | Moderate | Cost-effective, widely available, may require high purity |
| Helium | Low-hardenability alloys requiring high hardness | Very Fast | High cost, low distortion, approaches oil quenching rates |
| Argon | Highly reactive metals like titanium alloys | Slow | Completely inert, prevents surface reactions, more expensive |
| Hydrogen | Large parts or poor hardenability steels | Fastest | Highest cost, flammable, risk of hydrogen embrittlement |
Struggling to select the right gas for your heat treatment process? KINTEK specializes in advanced high-temperature furnace solutions, including Muffle, Tube, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems. With exceptional R&D and in-house manufacturing, we offer deep customization to precisely match your unique experimental needs, ensuring optimal cooling rates and material properties. Contact us today to discuss how our expertise can enhance your lab's efficiency and results!
Visual Guide
Related Products
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Molybdenum Vacuum Heat Treat Furnace
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
People Also Ask
- Where are vacuum furnaces used? Critical Applications in Aerospace, Medical, and Electronics
- What is the heat treatment in a vacuum furnace? Achieve Superior Metallurgical Properties
- What is the process of vacuum heat treatment? Achieve Superior Metallurgical Properties
- How does vacuum heat treatment improve mechanical properties of metals? Enhance Strength and Durability
- How does a vacuum furnace prevent heat transfer and contamination? Achieve Ultimate Material Purity



















