For optimal performance and lifespan, Molybdenum Disilicide (MoSi₂) heating elements must be operated in either an oxidizing atmosphere, such as air, or a chemically inert gas environment. They are highly susceptible to damage from active or reducing gases like hydrogen (H₂), chlorine (Cl₂), and sulfur dioxide (SO₂), which will cause rapid failure.
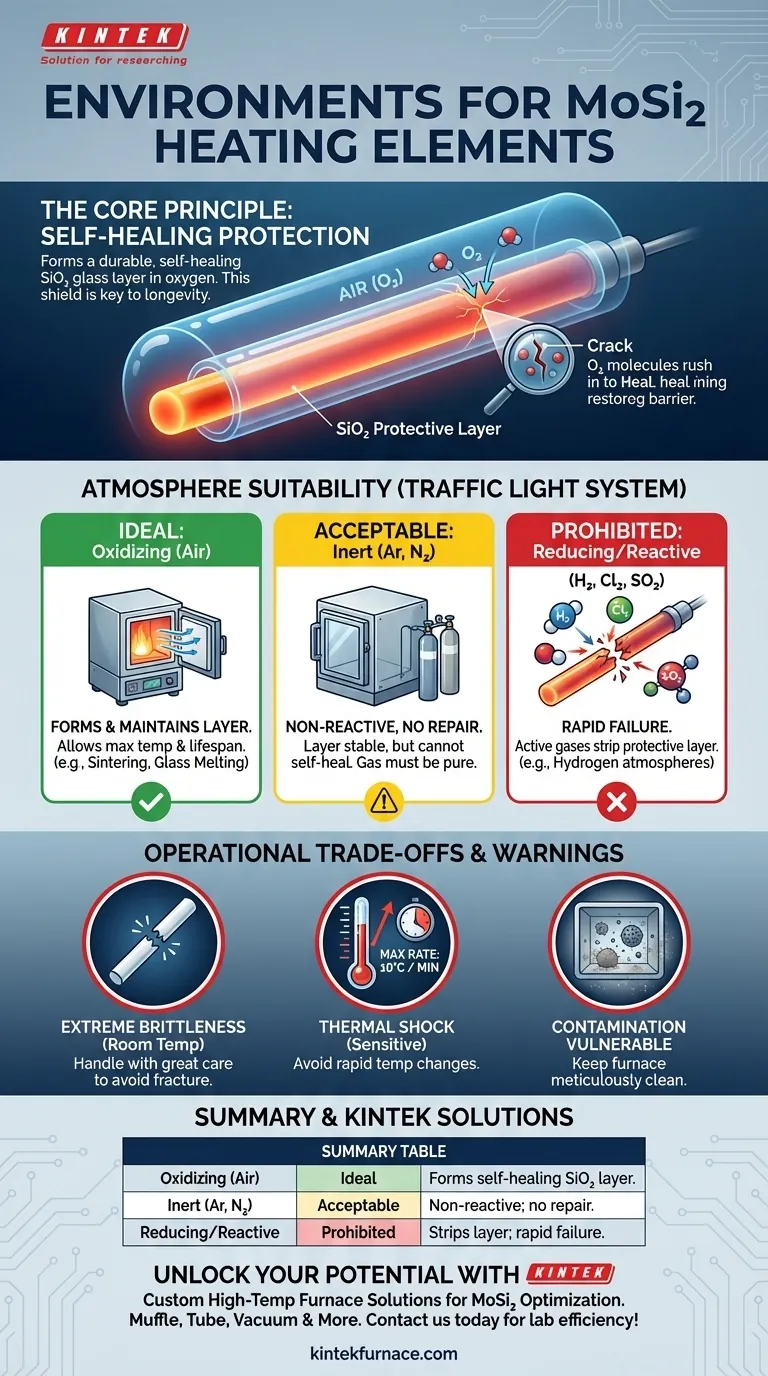
The core principle to understand is that MoSi₂ elements are designed to protect themselves. They rely on the presence of oxygen at high temperatures to form a durable, self-healing layer of silica glass (SiO₂) that shields them from further attack.
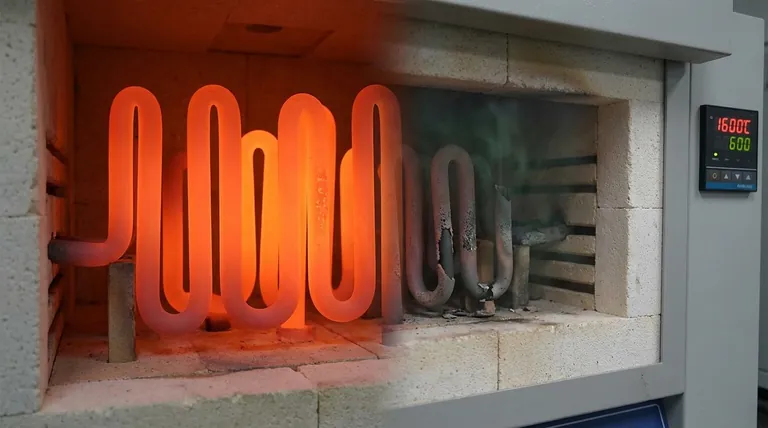
The Protective Mechanism of Oxidation
The exceptional high-temperature performance of a MoSi₂ element is not due to the material being inert, but rather due to its controlled, beneficial reaction with oxygen.
How the Protective Layer Forms
When heated, the silicon in the Molybdenum Disilicide compound reacts with oxygen from the surrounding air. This reaction forms a thin, non-porous, and self-healing layer of pure silica glass (SiO₂) on the element's surface.
This glass layer is the key to the element's longevity. It acts as a physical barrier, preventing the underlying MoSi₂ material from being consumed by further oxidation or chemical attack.
The "Self-Healing" Function
If a crack or flaw develops in the protective silica layer, the exposed MoSi₂ underneath will immediately react with available oxygen to "heal" the breach, restoring the protective barrier. This makes the elements exceptionally durable for continuous work in oxidizing atmospheres.
Permitted and Prohibited Atmospheres
Understanding which environments enable or destroy this protective layer is critical for successful operation.
Ideal: Oxidizing Atmospheres (Air)
Air is the most common and ideal operating environment. The abundant oxygen ensures the constant maintenance and repair of the protective SiO₂ layer, allowing the element to reach its maximum temperature and lifespan.
Acceptable: Inert Atmospheres
Inert gases like Argon (Ar) or Nitrogen (N₂) are also suitable. Because these gases are non-reactive, they will not chemically attack the element or interfere with the pre-existing protective layer. However, they do not contribute to its repair.
Prohibited: Reducing & Reactive Gases
Operating MoSi₂ elements in certain atmospheres will lead to rapid and catastrophic failure. These include:
- Hydrogen (H₂)
- Chlorine (Cl₂)
- Sulfur Dioxide (SO₂)
These gases actively strip away the protective silica layer or react directly with the element material itself, causing it to degrade and break.
Understanding the Operational Trade-offs
While powerful, MoSi₂ elements have specific limitations that demand careful handling and process control to prevent premature failure.
Extreme Physical Brittleness
These elements are ceramic-based and are extremely brittle at room temperature. They must be handled with great care during shipping, installation, and furnace maintenance to avoid fracture.
Sensitivity to Thermal Shock
Rapid changes in temperature create internal stresses that can easily crack the element. A maximum heating or cooling rate of 10°C per minute is a critical operational guideline to prevent thermal shock.
Vulnerability to Contamination
Foreign materials inside the furnace can be a major source of failure. Substances from process materials, such as certain colorants or binders in zirconia, can vaporize and react with the element's surface at high temperatures, compromising the protective layer. Meticulous furnace cleanliness is essential.
Making the Right Choice for Your Process
The suitability of MoSi₂ elements is determined entirely by your furnace's atmosphere and operational discipline.
- If your primary focus is high-temperature processing in air (e.g., sintering ceramics, melting glass): MoSi₂ is the industry-standard choice, offering unmatched temperature capability and lifespan.
- If your process requires an inert gas atmosphere (e.g., Argon): These elements are an excellent option, provided your gas supply is pure and the furnace is free of contaminants.
- If your process involves any reactive or reducing gases (e.g., Hydrogen atmospheres): You must choose a different heating element technology, as MoSi₂ will be chemically destroyed in this environment.
Ultimately, understanding and controlling the chemical environment within your furnace is the key to unlocking the exceptional performance of MoSi₂ heating elements.
Summary Table:
| Environment Type | Suitability | Key Characteristics |
|---|---|---|
| Oxidizing (e.g., Air) | Ideal | Forms self-healing SiO₂ layer for protection and longevity |
| Inert (e.g., Argon, Nitrogen) | Acceptable | Non-reactive; does not repair protective layer |
| Reducing/Reactive (e.g., Hydrogen, Chlorine) | Prohibited | Causes rapid failure by stripping protective layer |
Unlock the full potential of your high-temperature processes with KINTEK's advanced furnace solutions! Leveraging exceptional R&D and in-house manufacturing, we provide diverse laboratories with tailored high-temperature furnace systems, including Muffle, Tube, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems. Our strong deep customization capability ensures precise alignment with your unique experimental requirements, such as optimizing MoSi2 heating element performance. Contact us today to discuss how we can enhance your lab's efficiency and reliability!
Visual Guide
Related Products
- Silicon Carbide SiC Thermal Heating Elements for Electric Furnace
- Molybdenum Vacuum Heat Treat Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Laboratory Muffle Oven Furnace with Bottom Lifting
People Also Ask
- What are the advantages of using high purity green silicon carbide powder in heating elements? Boost Efficiency and Lifespan
- What makes SIC heating elements superior for high-temperature applications? Unlock Efficiency and Durability
- What are the properties and applications of silicon carbide (SiC)? Unlock High-Temperature Performance
- What are the properties and capabilities of Silicon Carbide (SiC) as a heating element? Unlock Extreme Heat and Durability
- What is the maximum temperature silicon carbide heating elements can withstand? Key Factors for Longevity and Performance



















