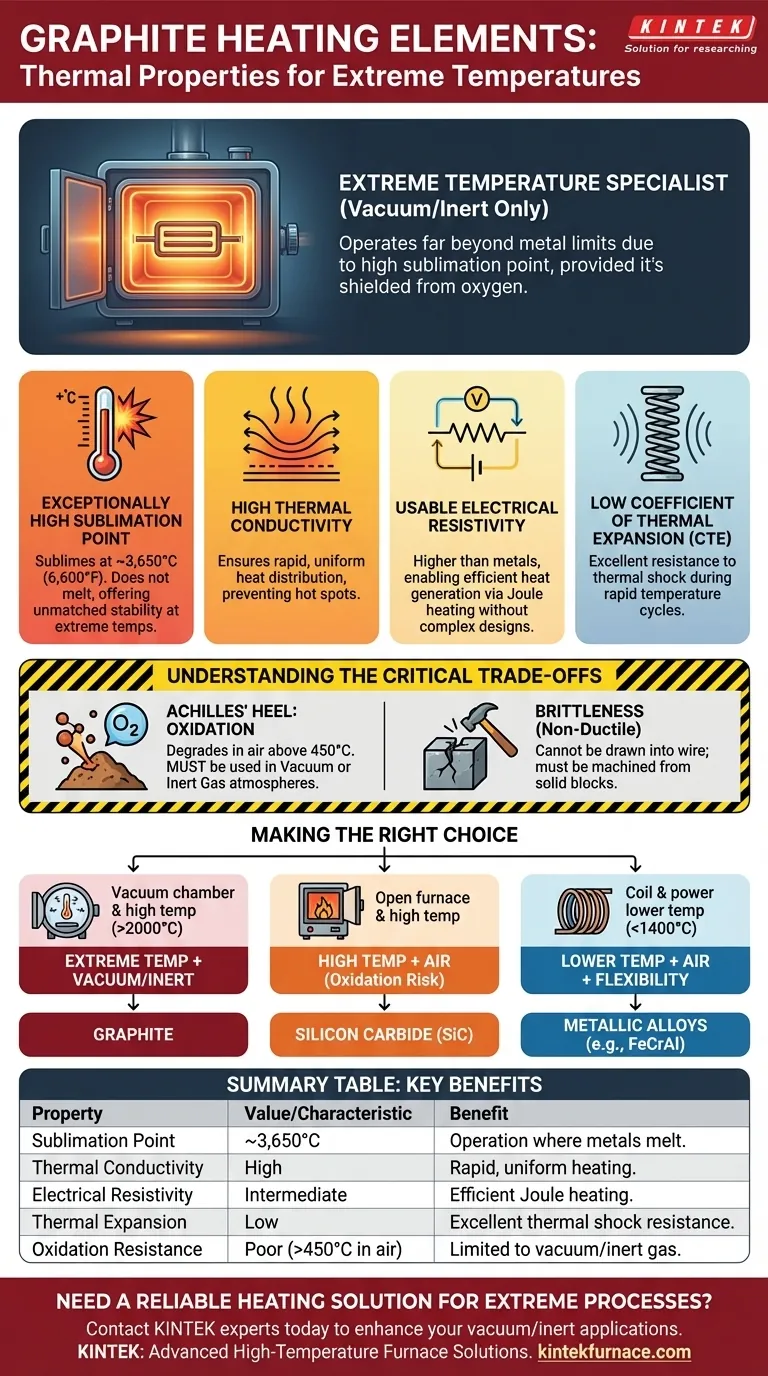
At its core, graphite's suitability as a heating element comes from its extremely high sublimation point, high thermal conductivity, and a level of electrical resistivity that effectively generates heat. These properties allow it to operate reliably at temperatures far beyond the limits of most metals, provided it is used in a non-oxidizing environment like a vacuum or inert gas furnace.
Graphite is not a universally ideal heating material; it is a specialist. Its exceptional performance at extreme temperatures is entirely dependent on being shielded from oxygen, making it the premier choice for vacuum and inert atmosphere furnaces but unsuitable for heating in open air.

The Core Thermal & Electrical Properties of Graphite
To understand why graphite is used in demanding applications like metal sintering and copper brazing, we must look at its unique combination of properties.
Exceptionally High Temperature Stability
Graphite does not melt at atmospheric pressure; instead, it sublimes (turns from a solid directly to a gas) at an incredibly high temperature, around 3,650°C (6,600°F).
This thermal stability allows graphite heating elements to operate in environments that would melt or destroy conventional metallic elements.
High Thermal Conductivity
Graphite exhibits high thermal conductivity, meaning it transfers heat very efficiently.
This property is critical for ensuring uniform temperature distribution within a furnace, preventing hot spots and allowing for rapid, even heating of the material being processed.
Usable Electrical Resistivity
While graphite is a good electrical conductor, its resistivity is significantly higher than that of metals like copper.
This intermediate resistivity is a key advantage. It is low enough to allow current to flow easily but high enough to generate substantial heat through resistance (Joule heating) without requiring unmanageably large or complex element designs.
Low Coefficient of Thermal Expansion (CTE)
Graphite expands and contracts very little when heated and cooled. This low thermal expansion gives it excellent resistance to thermal shock.
During rapid temperature cycles, elements with high CTE can crack or break due to internal stress. Graphite's stability minimizes this risk, contributing to a longer service life in demanding cyclic operations.
Understanding the Critical Trade-offs
No material is perfect. Graphite's unique strengths are balanced by significant limitations that define where and how it can be used.
The Achilles' Heel: Oxidation
Graphite's most significant weakness is its poor resistance to oxidation.
In the presence of air (oxygen), graphite begins to oxidize and degrade at temperatures as low as 450°C (842°F). This makes it entirely unsuitable for high-temperature applications in an open atmosphere. Its use is restricted to vacuum furnaces or furnaces filled with an inert gas like argon or nitrogen.
Mechanical Properties: Strength vs. Brittleness
Graphite has high compressive strength but is a brittle material, meaning it lacks ductility.
Unlike metallic heating element alloys, graphite cannot be drawn into a wire or easily formed. Elements must be carefully machined from large, solid blocks of graphite, which impacts the complexity and cost of the final design.
The Graphite vs. Silicon Carbide (SiC) Decision
When choosing a non-metallic heating element, the most common alternative is Silicon Carbide (SiC).
SiC also offers high thermal conductivity and excellent thermal shock resistance. However, its key advantage is its superior oxidation resistance, allowing it to operate at high temperatures in air. This makes SiC the default choice for applications that cannot be performed in a vacuum.
Making the Right Choice for Your Application
Selecting the correct heating element material requires matching its properties directly to the operating environment and performance goals of your process.
- If your primary focus is reaching extreme temperatures (>2000°C) in a vacuum or inert atmosphere: Graphite is the superior and often only viable choice due to its unmatched sublimation point.
- If your primary focus is high-temperature heating in an open-air environment: Silicon Carbide (SiC) is the required material because of its inherent ability to resist oxidation.
- If your primary focus is design flexibility and lower-temperature heating (typically <1400°C) in air: Ductile metallic alloys like FeCrAl (e.g., Kanthal) are often the most practical and cost-effective solution.
Ultimately, choosing the right heating element is about understanding the environment first and the material second.
Summary Table:
| Property | Value / Characteristic | Key Benefit for Heating Elements |
|---|---|---|
| Sublimation Point | ~3,650°C (6,600°F) | Enables operation at extreme temperatures where metals melt. |
| Thermal Conductivity | High | Ensures rapid, uniform heating and prevents hot spots. |
| Electrical Resistivity | Intermediate (higher than metals) | Efficiently generates heat via Joule heating without complex designs. |
| Thermal Expansion (CTE) | Low | Provides excellent thermal shock resistance for long service life. |
| Oxidation Resistance | Poor (degrades above 450°C in air) | Limits use to vacuum or inert gas atmospheres. |
Need a reliable heating solution for extreme-temperature processes?
Leveraging exceptional R&D and in-house manufacturing, KINTEK provides diverse laboratories with advanced high-temperature furnace solutions. Our product line, including Muffle, Tube, and Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems, is complemented by our strong deep customization capability to precisely meet unique experimental requirements.
Contact our experts today to discuss how our graphite heating elements and furnace systems can enhance your high-temperature applications in vacuum or inert environments.
Visual Guide
Related Products
- Silicon Carbide SiC Thermal Heating Elements for Electric Furnace
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
- Laboratory Muffle Oven Furnace with Bottom Lifting
- Split Multi Heating Zone Rotary Tube Furnace Rotating Tube Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
People Also Ask
- What are the properties and applications of silicon carbide (SiC)? Unlock High-Temperature Performance
- Why is silicon carbide resistant to chemical reactions in industrial furnaces? Unlock Durable High-Temp Solutions
- What makes SIC heating elements superior for high-temperature applications? Unlock Efficiency and Durability
- What is the maximum temperature silicon carbide heating elements can withstand? Key Factors for Longevity and Performance
- What are the properties and capabilities of Silicon Carbide (SiC) as a heating element? Unlock Extreme Heat and Durability














