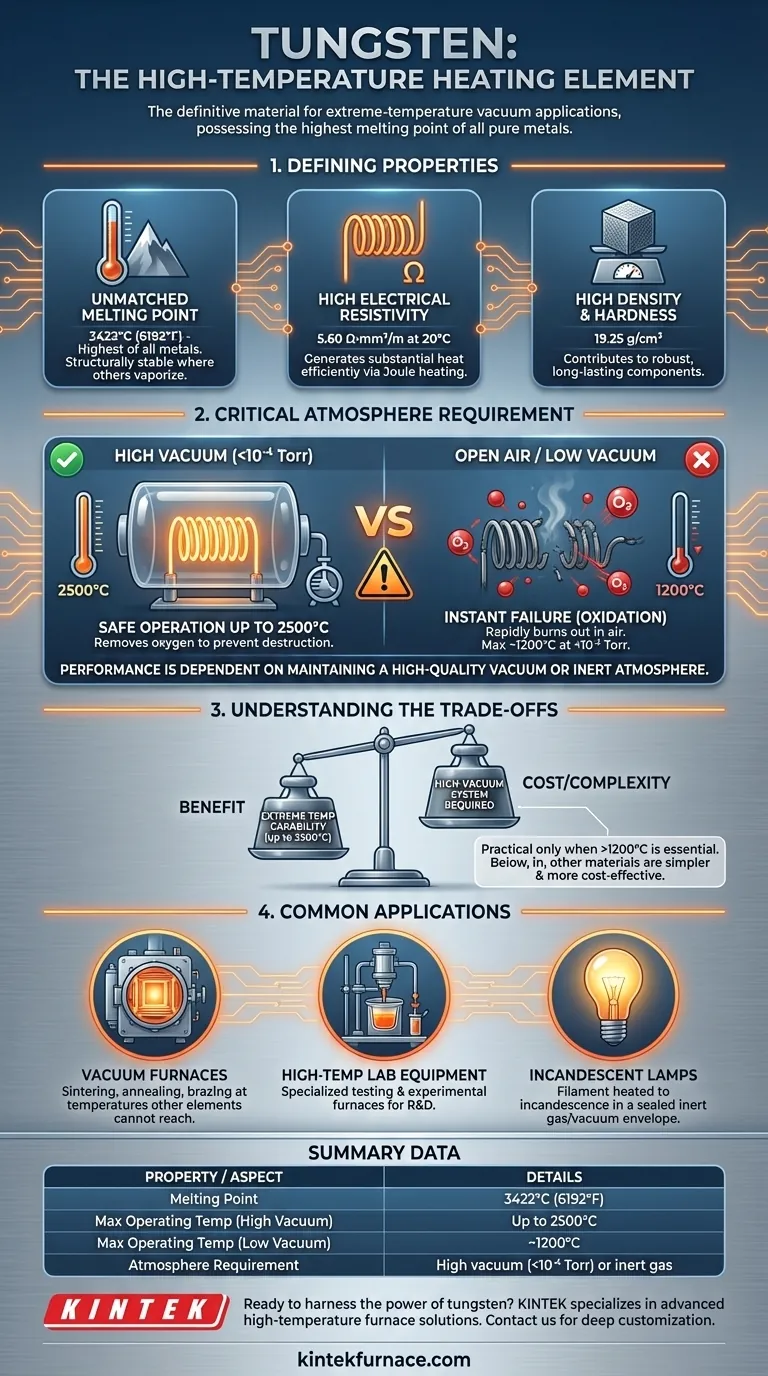
Of all pure metals, tungsten possesses the highest melting point, making it the definitive material for constructing heating elements intended for high-temperature vacuum applications. Its key properties are an extremely high melting point (3422°C), sufficient electrical resistivity (5.60 Ω·mm²/m at 20°C), and high density. This combination allows it to operate reliably at temperatures up to 2500°C, but only under specific atmospheric conditions.
Tungsten is unmatched for heating in extreme-temperature environments, but its performance is not inherent—it is entirely dependent on maintaining a high-quality vacuum or inert atmosphere to prevent its rapid failure.

The Defining Properties of Tungsten
The suitability of tungsten as a heating element is not due to a single property, but the combination of several thermal and electrical characteristics.
Unmatched Melting Point
Tungsten's melting point of 3422°C (6192°F) is the highest of all metals. This fundamental property is the primary reason it is chosen for applications that operate far above the limits of common materials like nickel-chromium or iron-chromium-aluminum alloys.
This allows it to remain solid and structurally stable at temperatures where most other conductive materials would have already vaporized or melted.
High Electrical Resistivity
For a material to function as a heating element, it must resist the flow of electricity, thereby generating heat (a principle known as Joule heating).
Tungsten's electrical resistivity is high enough to generate substantial heat efficiently without requiring excessively high currents. This makes the design of power control systems more practical.
Physical Density and Hardness
With a density of 19.25 g/cm³, tungsten is one of the densest elements. While not directly related to heat generation, its hardness and density contribute to its use in robust, long-lasting components, provided it is handled correctly.
Why Tungsten Requires a Controlled Atmosphere
The single most important factor governing the use of tungsten heating elements is the environment in which they operate. Using tungsten in the wrong atmosphere will lead to immediate and catastrophic failure.
The Problem of Oxidation
Tungsten oxidizes very rapidly in the presence of air or other oxygen-bearing gases at elevated temperatures. This oxidation process destroys the metal, causing the element to burn out almost instantly.
Therefore, tungsten heating elements cannot be operated in an open-air environment.
The Role of High Vacuum
To reach its maximum potential operating temperature of approximately 2500°C, tungsten must be placed in a high-vacuum environment. The references specify a vacuum level of less than 10⁻⁴ Torr.
This high vacuum effectively removes the oxygen molecules that would otherwise react with and destroy the hot tungsten.
Limitations in Low Vacuum
Even a slight degradation in vacuum quality has a significant impact on the maximum safe operating temperature.
At a lower vacuum level of less than 10⁻² Torr, the maximum recommended operating temperature for tungsten drops sharply to around 1200°C. This demonstrates the critical relationship between atmospheric purity and thermal performance.
Understanding the Trade-offs
Choosing tungsten is a decision with clear benefits and strict operational requirements. Understanding these trade-offs is essential for successful implementation.
Temperature Capability vs. Environmental Cost
The primary trade-off is performance versus complexity. To unlock tungsten's 2500°C capability, you must design, operate, and maintain a high-vacuum system, which adds significant cost and complexity to any furnace or process.
Limited Use Below 1200°C
While tungsten can operate at lower temperatures, it is often not the most practical choice. In the range below 1200°C, other heating element materials (like Kanthal) can operate in air without the need for a vacuum, making them far simpler and more cost-effective.
Common Applications for Tungsten Elements
Given its properties and requirements, tungsten is used in applications where extreme heat is a necessity and a controlled atmosphere is already part of the process.
Vacuum Furnaces
This is the most common industrial application. Tungsten elements are used to heat materials for processes like sintering, annealing, and brazing at temperatures that other elements cannot achieve.
High-Temperature Laboratory Equipment
Research and development labs use tungsten elements in specialized testing equipment and experimental furnaces to study materials and phenomena under extreme thermal conditions.
Incandescent Lamp Filaments
The classic example is the filament in a traditional light bulb. An electric current heats a tiny tungsten coil to incandescence (glowing hot), producing light within a sealed, inert-gas-filled or vacuum glass envelope.
Making the Right Choice for Your Goal
Your decision to use tungsten must be based on your specific temperature and atmospheric requirements.
- If your primary focus is achieving the highest possible process temperatures (1600°C to 2500°C): Tungsten is the superior choice, but you must commit to designing and maintaining a high-vacuum furnace environment.
- If your primary focus is operating in an air atmosphere at any temperature: Tungsten is completely unsuitable and will fail instantly; you must select an oxidation-resistant material instead.
- If your primary focus is general-purpose heating below 1200°C: Tungsten is often an overly complex and expensive solution compared to other elements that do not require a vacuum.
Ultimately, tungsten is the expert's choice for extreme heat, delivering unparalleled performance when its strict environmental needs are met.
Summary Table:
| Property / Aspect | Details |
|---|---|
| Melting Point | 3422°C (6192°F) |
| Electrical Resistivity | 5.60 Ω·mm²/m at 20°C |
| Density | 19.25 g/cm³ |
| Max Operating Temp (High Vacuum) | Up to 2500°C |
| Max Operating Temp (Low Vacuum) | ~1200°C |
| Key Applications | Vacuum furnaces, high-temperature lab equipment, incandescent lamps |
| Atmosphere Requirement | High vacuum (<10⁻⁴ Torr) or inert gas to prevent oxidation |
Ready to harness the power of tungsten for your high-temperature applications? At KINTEK, we specialize in advanced high-temperature furnace solutions tailored to your needs. Leveraging our exceptional R&D and in-house manufacturing, we offer a diverse product line including Muffle, Tube, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems. With strong deep customization capabilities, we precisely meet unique experimental requirements, ensuring optimal performance and reliability. Don't let complexity hold you back—contact us today to discuss how we can elevate your lab's capabilities with our expert solutions!
Visual Guide
Related Products
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Laboratory Muffle Oven Furnace with Bottom Lifting
People Also Ask
- How do vacuum furnaces contribute to long-term cost savings? Reduce Costs with Efficiency and Quality
- Why might a vacuum furnace maintain vacuum during cooling? Protect Workpieces from Oxidation and Control Metallurgy
- How are parts loaded into a vacuum furnace? Ensure Precision and Efficiency in Your Process
- How does vacuum heat treatment reduce workpiece deformation? Achieve Superior Dimensional Stability
- What role does a vacuum sintering furnace play in the formation of the 'core-rim' structure in Ti(C,N)-FeCr cermets?



















