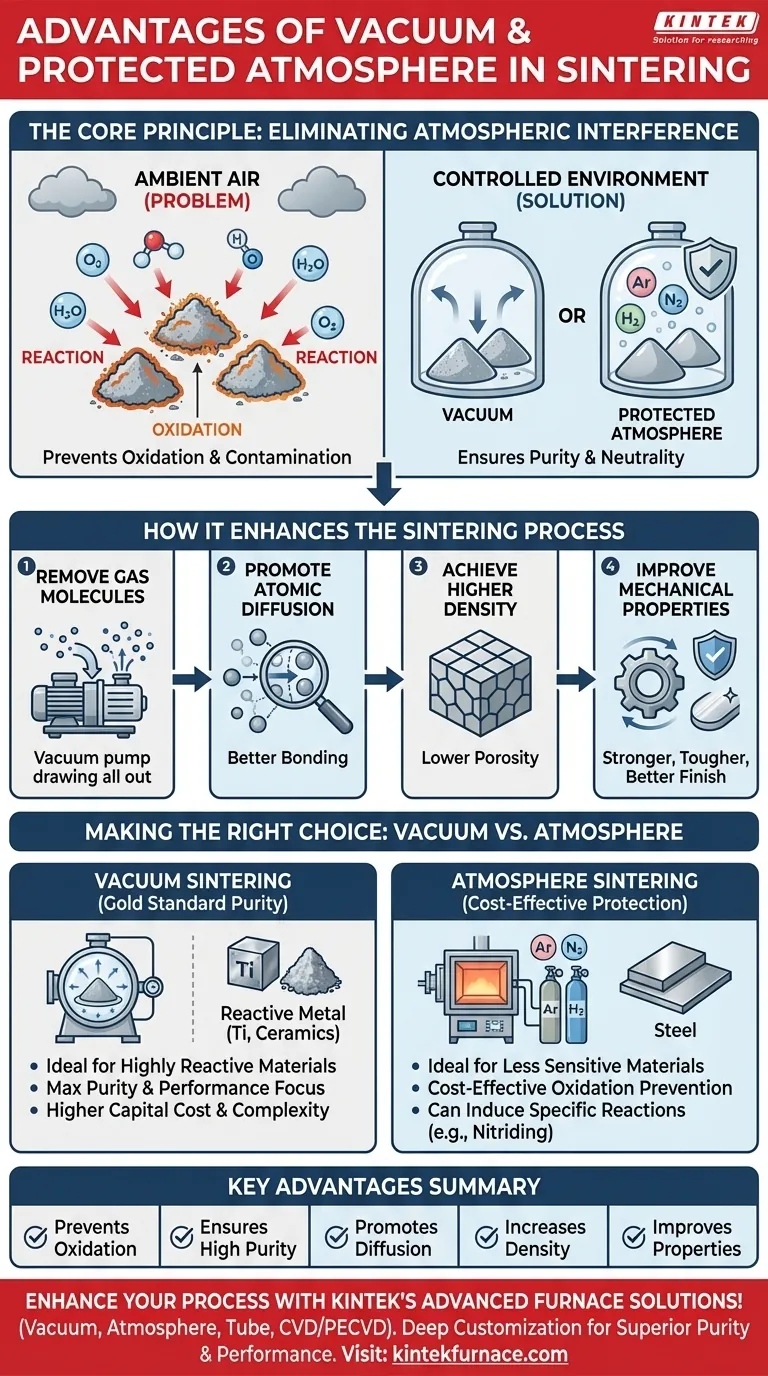
In short, using a vacuum or protected atmosphere during sintering is essential for preventing chemical reactions like oxidation that degrade material quality. By removing reactive atmospheric gases, these controlled environments ensure the final product achieves higher purity, superior density, and significantly improved mechanical properties, such as strength and toughness.
Moving the sintering process into a controlled environment is not merely a precaution; it is a fundamental process enhancement. It eliminates atmospheric interference to give you direct control over the atomic-level interactions that define your final material's quality and performance.
The Core Principle: Eliminating Atmospheric Interference
Sintering occurs at extremely high temperatures, making materials highly susceptible to reacting with ambient air. A vacuum or controlled gas atmosphere directly counteracts this vulnerability.
Preventing Oxidation and Contamination
At sintering temperatures, oxygen is highly reactive and will readily form oxides on the surface of powder particles. This creates impurity layers that inhibit the bonding process.
A controlled environment removes oxygen and other contaminants like water vapor, preventing these unwanted chemical reactions from ever occurring.
Ensuring Maximum Material Purity
For applications involving sensitive materials like titanium alloys, medical implants, or advanced ceramics, even trace amounts of impurities can be detrimental.
A high-vacuum environment is the most effective way to eliminate virtually all foreign gases, ensuring the sintered part meets stringent purity requirements.
Creating a Neutral Environment
Beyond just oxygen, other gases can cause unwanted reactions. For example, certain atmospheres can lead to the decarburization or carburization of steels, altering their intended properties.
A vacuum is fundamentally neutral, providing an environment where the material being sintered will not react with its surroundings.
How a Controlled Environment Enhances Sintering
By removing atmospheric variables, the sintering process itself becomes more efficient and effective, leading to a measurably better final product.
Promoting Atomic Diffusion
Sintering works by encouraging atoms from individual powder particles to migrate, or diffuse, across boundaries to form solid bonds.
Removing gas molecules that physically occupy space between particles and react with surfaces facilitates this atomic diffusion. This allows the particles to bond more effectively, which is the primary mechanism for densification.
Achieving Higher Density and Lower Porosity
When gases are trapped between powder particles during densification, they form voids or pores in the final material. These pores are structural weak points.
By conducting the process in a vacuum, trapped gases are eliminated, resulting in a product with higher density and significantly lower porosity.
Improving Mechanical Properties
The direct result of higher density and purity is a mechanically superior component.
A dense, non-porous structure is inherently stronger and tougher. Furthermore, a clean surface free of oxides leads to better fatigue life and a higher quality finish, often reducing the need for secondary machining.
Understanding the Trade-offs: Vacuum vs. Atmosphere
While both methods protect the material, they are not interchangeable. The choice between a vacuum and a specific gas atmosphere depends on the material, the desired outcome, and operational constraints.
When to Use Vacuum Sintering
Vacuum is the gold standard for purity. It is the preferred choice for highly reactive materials or when the absolute best mechanical properties are non-negotiable.
It creates the most neutral environment possible, making it ideal for materials where any side reaction could be catastrophic to performance.
When to Use Atmosphere Sintering
Atmosphere sintering involves backfilling the furnace with a controlled gas like argon, nitrogen, or hydrogen. This is a cost-effective way to prevent oxidation for less sensitive materials.
While it protects from oxygen, the gas itself can have slight interactions with the material. In some cases, this is intentional, such as using a nitrogen atmosphere to form nitrides.
The Cost and Complexity Factor
Vacuum furnaces are typically more complex and carry a higher capital cost than atmosphere furnaces. Achieving and maintaining a high vacuum requires robust pumping systems and seals.
Therefore, atmosphere sintering is often chosen for high-volume production where the extreme purity of a vacuum is not strictly necessary.
Making the Right Choice for Your Material
Your choice of environment should be driven by the end-use requirements of your component. A clear understanding of your goals will dictate the most appropriate and cost-effective path.
- If your primary focus is maximum purity and performance: Use vacuum sintering, especially for reactive materials like titanium, refractory metals, or high-performance ceramics.
- If your primary focus is cost-effective oxidation prevention: Use atmosphere sintering with an inert gas like argon for most steels and non-ferrous alloys.
- If your primary focus is inducing a specific chemical reaction: Use a reactive atmosphere, such as nitrogen for nitriding or hydrogen for oxide reduction.
Ultimately, selecting the right environment is about matching the process to the precise material properties your application demands.
Summary Table:
| Advantage | Description |
|---|---|
| Prevents Oxidation | Eliminates oxygen and contaminants to avoid surface impurities and degradation. |
| Ensures High Purity | Removes foreign gases for sensitive materials like titanium alloys and medical implants. |
| Promotes Atomic Diffusion | Facilitates better bonding between powder particles by reducing gas interference. |
| Increases Density | Reduces porosity by eliminating trapped gases, leading to stronger structures. |
| Improves Mechanical Properties | Enhances strength, toughness, and fatigue life with a clean, dense finish. |
| Cost-Effective Options | Atmosphere sintering with inert gases offers oxidation prevention for less sensitive materials. |
Enhance your sintering process with KINTEK's advanced furnace solutions! Leveraging exceptional R&D and in-house manufacturing, we provide diverse laboratories with high-temperature furnaces like Muffle, Tube, Rotary, Vacuum & Atmosphere, and CVD/PECVD Systems. Our strong deep customization capability ensures we precisely meet your unique experimental needs, delivering superior purity, density, and performance for materials like titanium alloys and ceramics. Contact us today to discuss how we can optimize your sintering environment and achieve your material goals!
Visual Guide
Related Products
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- Dental Porcelain Zirconia Sintering Ceramic Vacuum Press Furnace
- 600T Vacuum Induction Hot Press Vacuum Heat Treat and Sintering Furnace
People Also Ask
- What is an atmosphere protection muffle furnace? Unlock Precise Heat Treatment in Controlled Environments
- What are the primary inert gases used in vacuum furnaces? Optimize Your Heat Treatment Process
- What are the key features of an atmosphere box furnace? Unlock Precise Heat Processing in Controlled Environments
- How does the pressure range change under vacuum conditions in an atmosphere box furnace? Explore Key Shifts for Material Processing
- What are the development prospects of atmosphere box furnaces in the aerospace industry? Unlock Advanced Material Processing for Aerospace Innovation



















