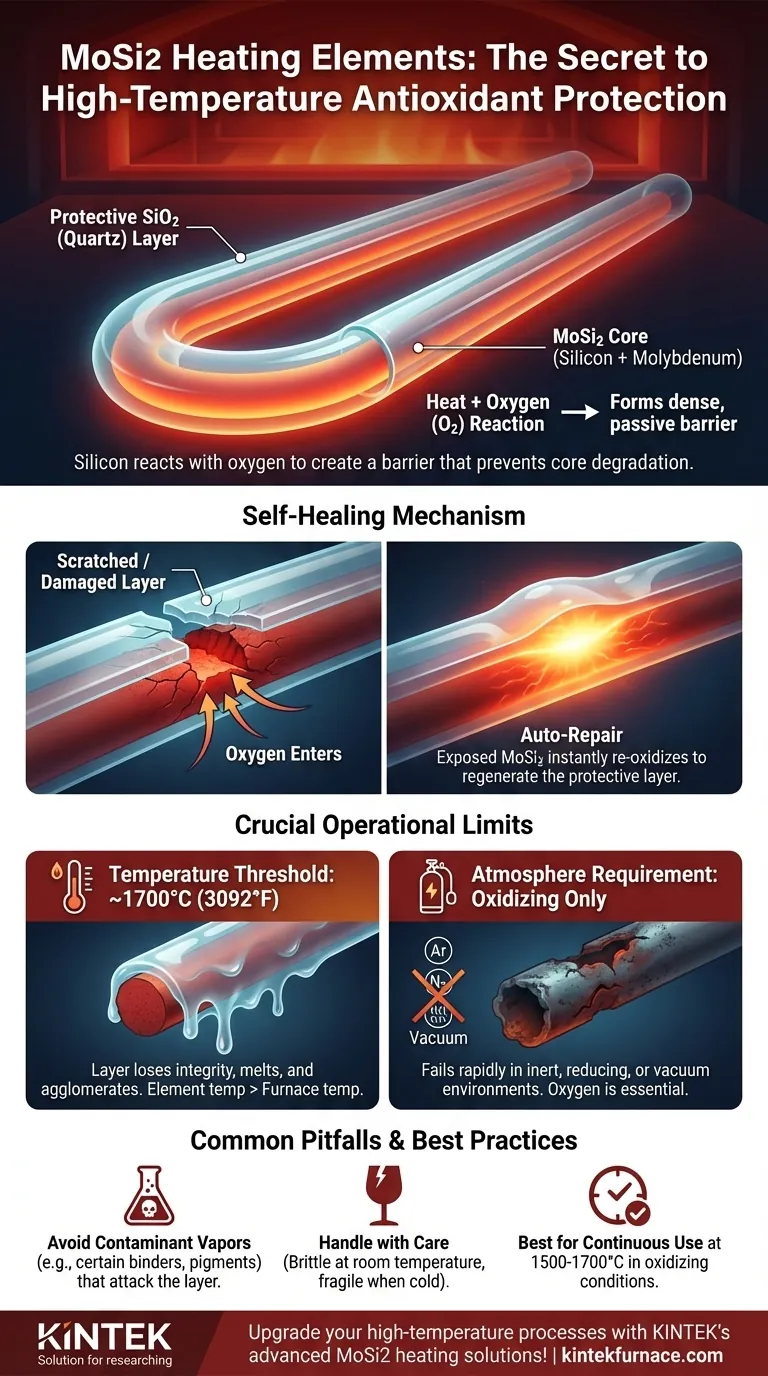
The high-temperature antioxidant property of Molybdenum Disilicide (MoSi2) heating elements stems from a remarkable chemical reaction on their surface. When heated in an oxidizing atmosphere, the silicon within the element material reacts with oxygen to form a thin, dense, and non-porous protective layer of quartz (silicon dioxide, SiO2). This passive glass-like layer acts as a physical barrier, preventing oxygen from reaching and degrading the underlying MoSi2 core, ensuring the element's longevity at extreme temperatures.
The protective SiO2 layer is the key to MoSi2's durability, but it is not infallible. Its effectiveness is entirely dependent on maintaining the right operating conditions—specifically, an oxidizing atmosphere and keeping temperatures below the layer's melting point of approximately 1700°C.

The Core Mechanism: How Oxidation Creates Protection
To truly understand the reliability of MoSi2 elements, we must look at how this protective layer forms, functions, and even repairs itself.
The Formation of the SiO2 Layer
At high temperatures, the silicon (Si) component of the MoSi2 alloy is highly reactive with oxygen in the surrounding atmosphere. This reaction forms a stable, glass-like coating of silicon dioxide (SiO2).
This process is inherent to the material itself, requiring only heat and oxygen to activate.
A Stable Ceramic Barrier
The resulting SiO2 layer is dense and chemically inert. It effectively seals the surface of the heating element.
This barrier physically prevents further, more destructive oxidation of the molybdenum and silicon components, which would otherwise cause the element to degrade and fail.
The Self-Healing Phenomenon
A key advantage of this mechanism is its ability to "auto-repair." If the protective SiO2 layer is scratched or damaged during operation, the newly exposed hot MoSi2 material will immediately react with atmospheric oxygen.
This reaction instantly regenerates the protective layer in the damaged area, effectively healing the breach and restoring the element's defense against oxidation. This is why these elements are exceptionally well-suited for continuous work.
Understanding the Operational Limits
While robust, the protective mechanism has critical boundaries. Operating outside of these conditions will lead to premature element failure.
The 1700°C Temperature Threshold
The primary limitation is temperature. The protective SiO2 layer has a melting point around 1700°C (3092°F).
Above this temperature, the layer loses its structural integrity, melting and agglomerating into small drops. This exposes the core material to rapid oxidation and damage. While regeneration can occur, frequent operation above this threshold significantly shortens the element's life.
Element vs. Furnace Temperature
It is critical to distinguish between the furnace's internal temperature and the element's surface temperature. The heating element itself will always be significantly hotter than the chamber it is heating.
A furnace operating near 1600-1700°C may have element surface temperatures approaching 1800-1900°C, pushing the SiO2 layer past its stable limit.
The Absolute Need for an Oxidizing Atmosphere
The entire protective mechanism relies on the availability of oxygen.
Using MoSi2 elements in a reducing, inert, or vacuum atmosphere prevents the formation and regeneration of the SiO2 layer. Without this protection, the element will fail very quickly at high temperatures.
Common Pitfalls and Trade-offs
Proper use is essential for maximizing the lifespan and performance of MoSi2 elements.
Risk of Contamination
The integrity of the SiO2 layer can be compromised by chemical reactions with contaminants.
Materials like certain colored pigments or binders used on zirconia can release vapors that attack the protective layer. Ensuring proper furnace maintenance and drying of any materials being processed is crucial to prevent this chemical degradation.
Brittleness at Room Temperature
Like many ceramic-based materials, MoSi2 is brittle and fragile at room temperature. Care must be taken during installation and handling to avoid physical shock or stress.
Manufacturers often use special molding processes for the joints to improve impact resistance, but the heating sections remain susceptible to damage when cold.
Making the Right Choice for Your Application
Understanding these characteristics allows you to determine if MoSi2 elements are the correct choice for your specific high-temperature needs.
- If your primary focus is continuous operation between 1500°C and 1700°C: MoSi2 is an excellent choice, as its self-healing SiO2 layer provides superior longevity and reliability in an oxidizing atmosphere.
- If your process requires frequent cycling above 1700°C: Be aware that you are operating at the limit of the protective layer, which will likely degrade and shorten the element's overall lifespan.
- If you are working in a non-oxidizing (inert, reducing, or vacuum) atmosphere: MoSi2 elements are fundamentally unsuitable and will fail rapidly, as they cannot form their necessary protective layer.
- If you are heating materials that may release chemical vapors: You must ensure these vapors will not react with and compromise the SiO2 layer, or take steps to properly ventilate the furnace.
By managing the operating atmosphere and temperature, you can fully leverage the unique self-healing properties of MoSi2 for reliable, high-temperature performance.
Summary Table:
| Aspect | Details |
|---|---|
| Mechanism | Forms a protective SiO2 layer via reaction with oxygen, acting as a barrier against oxidation |
| Self-Healing | Automatically repairs scratches or damage by regenerating the SiO2 layer during operation |
| Temperature Limit | Effective up to ~1700°C; above this, the layer melts, leading to rapid degradation |
| Atmosphere Requirement | Requires oxidizing atmosphere (e.g., air) for layer formation and maintenance |
| Common Pitfalls | Brittle at room temperature, sensitive to contaminants, and unsuitable for non-oxidizing environments |
| Best Applications | Ideal for continuous use at 1500-1700°C in oxidizing conditions; avoid in inert, reducing, or vacuum atmospheres |
Upgrade your high-temperature processes with KINTEK's advanced MoSi2 heating solutions! Leveraging exceptional R&D and in-house manufacturing, we provide diverse laboratories with reliable, high-performance furnaces like Muffle, Tube, Rotary, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems. Our strong deep customization capability ensures precise solutions for your unique experimental needs, enhancing durability and efficiency. Contact us today to discuss how our tailored heating elements can optimize your operations and extend equipment lifespan!
Visual Guide
Related Products
- Silicon Carbide SiC Thermal Heating Elements for Electric Furnace
- Molybdenum Vacuum Heat Treat Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Laboratory Muffle Oven Furnace with Bottom Lifting
- Split Multi Heating Zone Rotary Tube Furnace Rotating Tube Furnace
People Also Ask
- What are the properties and capabilities of Silicon Carbide (SiC) as a heating element? Unlock Extreme Heat and Durability
- Why is silicon carbide resistant to chemical reactions in industrial furnaces? Unlock Durable High-Temp Solutions
- Why are silicon carbide heating elements essential in high-temperature industries? Unlock Reliable, Extreme Heat Solutions
- What is the maximum temperature silicon carbide heating elements can withstand? Key Factors for Longevity and Performance
- Why are SiC heating elements considered environmentally friendly? Discover Their Eco-Efficiency & Lifespan Insights



















