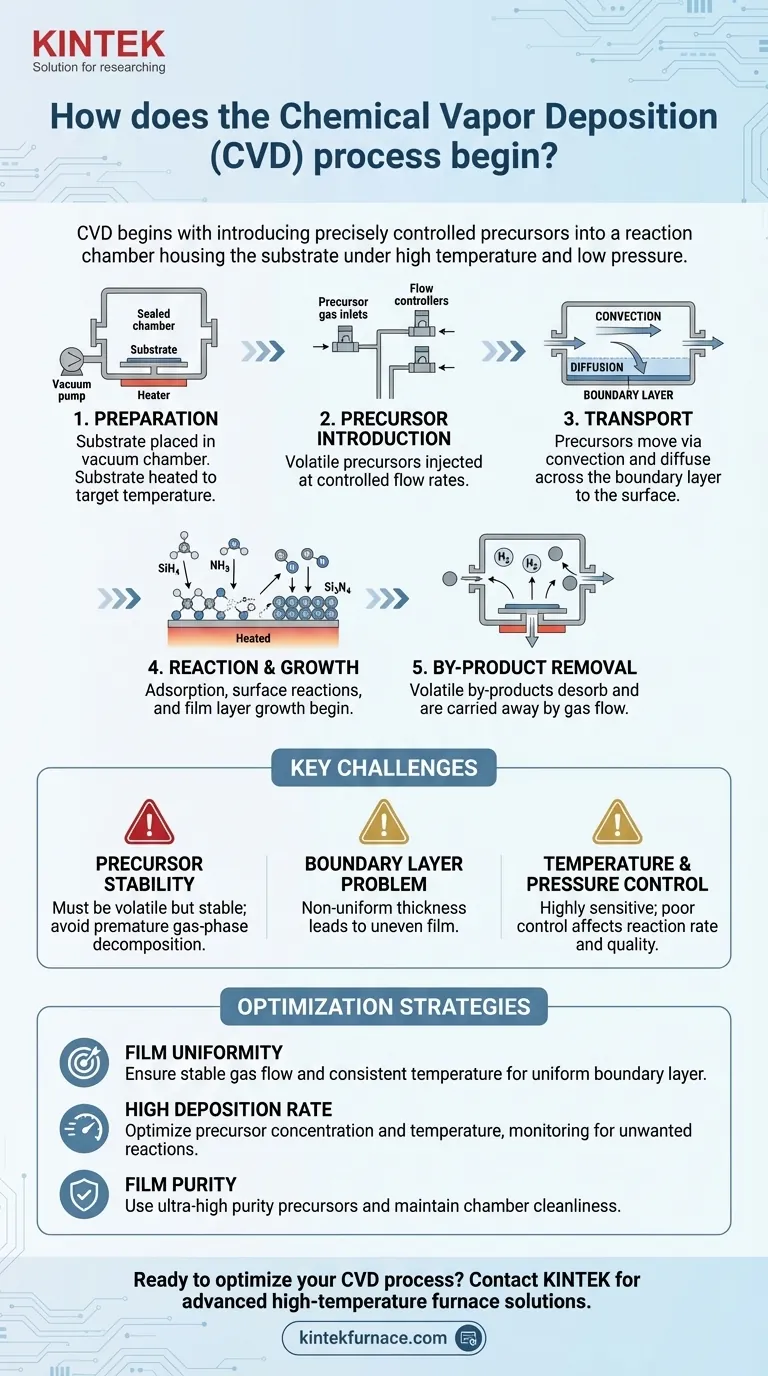
The Chemical Vapor Deposition (CVD) process begins by introducing precisely controlled gaseous or liquid reactants, known as precursors, into a reaction chamber that houses the target material, or substrate. These volatile precursors are selected because they contain the specific elements needed for the final film. The chamber itself is prepared under carefully managed conditions, such as high temperature and low pressure, to facilitate the upcoming reaction.
The start of CVD is not merely injecting a gas; it's about establishing a highly controlled environment. The initial steps are designed to transport volatile precursor molecules to a substrate, setting the stage for the chemical reactions that build a solid film, atom by atom.

The Foundational Stages of Deposition
To truly understand how CVD begins, we must look at the sequence of events that precede the actual film growth. It is a process of meticulous preparation and controlled transport.
Preparing the Substrate and Chamber
Before any reaction can occur, the environment must be perfected. The substrate—the material to be coated, such as a silicon wafer—is physically placed inside the reaction chamber.
The chamber is then sealed and often brought to a vacuum, well below atmospheric pressure. This removes contaminants and gives operators precise control over the atmosphere. The substrate is heated to a specific target temperature required to drive the chemical reaction.
Introducing the Precursors
This is the active start of the process. One or more precursors, which are volatile chemical compounds in gas or vaporized liquid form, are introduced into the chamber at a controlled flow rate.
Each precursor is chosen to contribute specific atoms to the final film. For example, to create a silicon nitride (Si₃N₄) film, precursors like silane (SiH₄) and ammonia (NH₃) might be used.
Transport to the Surface
Once inside the chamber, the precursor molecules do not instantly coat the substrate. They must first travel from the gas inlet to the substrate's surface.
This journey occurs in two primary ways. First, convection is the bulk movement of the gas, carrying the precursors throughout the chamber. As the gas approaches the substrate, a thin, stagnant "boundary layer" of gas forms. The precursors must then cross this layer via diffusion to finally reach the surface.
From Gas to Solid: The Reaction Cascade
The initial stages of precursor introduction and transport are designed to enable a cascade of chemical events that ultimately form the solid film.
Adsorption and Surface Reactions
When a precursor molecule reaches the substrate, it can "stick" to the hot surface in a process called adsorption.
Fueled by the thermal energy from the heated substrate, the adsorbed precursor molecules decompose or react with other precursors. This surface reaction is the core of CVD; it breaks chemical bonds, deposits the desired atoms onto the surface, and forms the new solid layer.
Film Growth and By-product Removal
The deposited atoms bond with the substrate and with each other, initiating the growth of a thin, uniform film. The process is designed to build this film layer by layer, sometimes molecule by molecule, ensuring high quality and control.
The chemical reactions also create unwanted molecules known as volatile by-products. These by-products desorb (un-stick) from the surface and are carried away by the continuous gas flow, exiting the chamber as exhaust.
Understanding the Key Challenges
The initial steps of CVD are critical, and several challenges must be managed to ensure a successful deposition. Getting the beginning wrong will compromise the entire result.
Precursor Choice and Stability
The choice of precursor is fundamental. It must be volatile enough to be transported as a gas but stable enough not to decompose prematurely in the gas phase. Unwanted gas-phase reactions can form particles that fall onto the substrate, creating defects in the film.
The Boundary Layer Problem
The stagnant boundary layer can act as a bottleneck, slowing the rate at which precursors reach the surface. If this layer is not uniform in thickness across the substrate, it will lead to a non-uniform film, where some areas are thicker than others.
Temperature and Pressure Control
The process is highly sensitive to temperature and pressure. If the temperature is too low, the surface reactions will not occur efficiently, leading to slow or no growth. If it is too high, precursors may react in the gas phase before ever reaching the surface, depleting the reactants and creating contaminating particles.
Making the Right Choice for Your Goal
The way you manage the beginning of the CVD process directly impacts the quality of your final film. Your focus should align with your primary objective.
- If your primary focus is film uniformity: Concentrate on creating a stable, predictable gas flow and maintaining an extremely consistent temperature across the entire substrate to ensure a uniform boundary layer.
- If your primary focus is a high deposition rate: Use higher precursor concentrations and optimal temperatures, but carefully monitor for the onset of gas-phase reactions that could degrade film quality.
- If your primary focus is film purity: Your first priority must be the use of ultra-high purity precursor gases and ensuring the absolute integrity and cleanliness of the reaction chamber.
Mastering the initial steps of precursor delivery and environmental control transforms CVD from a complex procedure into a predictable and powerful materials engineering tool.
Summary Table:
| Stage | Key Actions | Purpose |
|---|---|---|
| Preparation | Place substrate, vacuum chamber, heat substrate | Remove contaminants, set reaction conditions |
| Precursor Introduction | Inject volatile gases/vapors at controlled flow | Supply elements for film deposition |
| Transport | Convection and diffusion across boundary layer | Deliver precursors to substrate surface |
| Reaction | Adsorption, decomposition, surface reactions | Initiate solid film growth |
| By-product Removal | Desorption and exhaust of volatile by-products | Maintain purity and continuous deposition |
Ready to optimize your CVD process for superior thin films? At KINTEK, we leverage exceptional R&D and in-house manufacturing to provide advanced high-temperature furnace solutions tailored for your lab's needs. Our product line includes Muffle, Tube, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems, all backed by strong deep customization capabilities to precisely meet your unique experimental requirements. Contact us today to discuss how we can enhance your deposition outcomes with reliable, high-performance equipment!
Visual Guide
Related Products
- RF PECVD System Radio Frequency Plasma Enhanced Chemical Vapor Deposition
- Custom Made Versatile CVD Tube Furnace Chemical Vapor Deposition CVD Equipment Machine
- Inclined Rotary Plasma Enhanced Chemical Deposition PECVD Tube Furnace Machine
- Multi Heating Zones CVD Tube Furnace Machine for Chemical Vapor Deposition Equipment
- Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
People Also Ask
- What role does PECVD play in optical coatings? Essential for Low-Temp, High-Precision Film Deposition
- What are the main components of a PECVD system? Unlock Low-Temperature Thin Film Deposition
- How does plasma vapor deposition work? A Low-Temperature Solution for Advanced Coatings
- What gases are used in the PECVD system? Optimize Thin Film Deposition with Precise Gas Selection
- What is the second benefit of deposition within a discharge in PECVD? Enhance Film Quality with Ion Bombardment



















