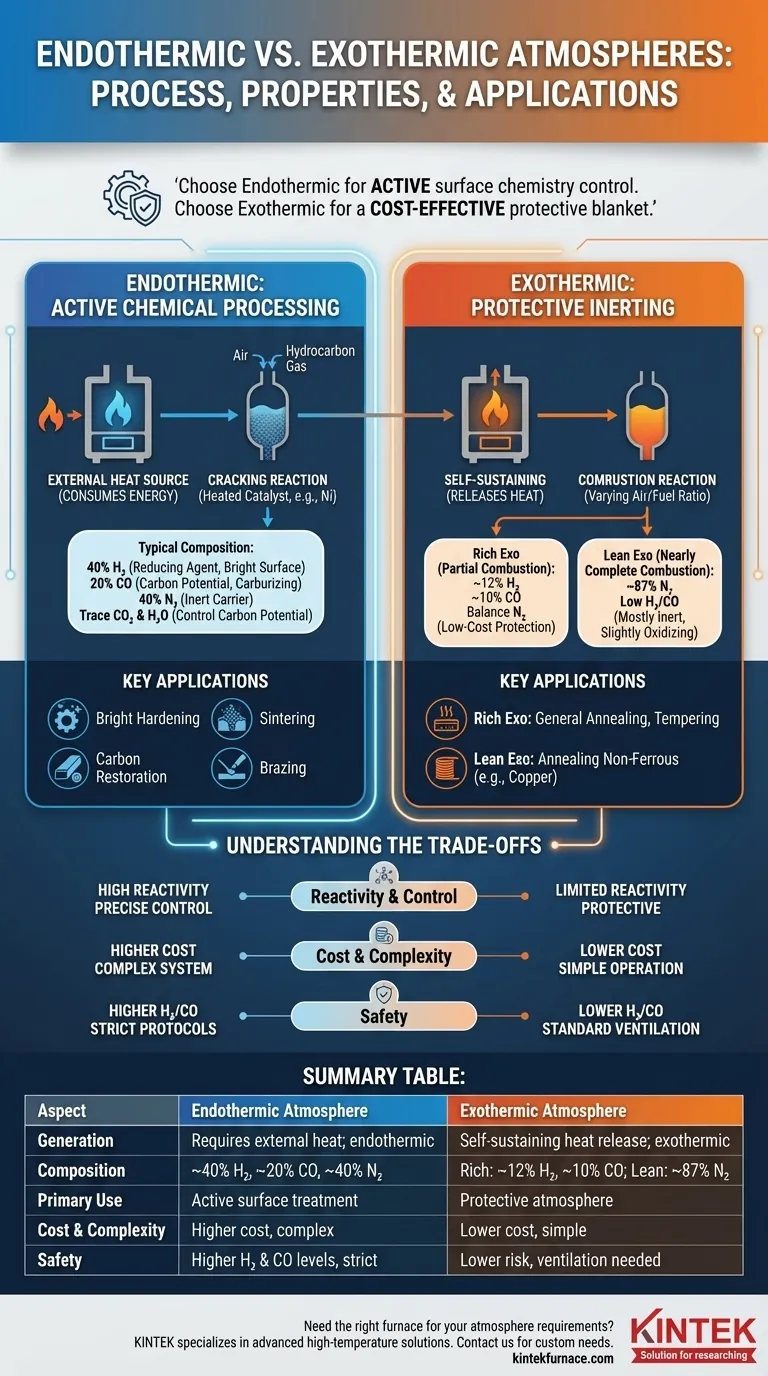
At its core, an endothermic atmosphere is a gas mixture created through a reaction that requires an external heat source, while an exothermic atmosphere is generated from a reaction that releases its own heat. This fundamental difference in generation dictates their composition and, consequently, their use in metallurgical heat treatment processes.
The choice between an endothermic and exothermic atmosphere comes down to your goal. Use endothermic gas when you need to actively control the surface chemistry of a metal, such as adding carbon. Use exothermic gas when you simply need a cost-effective, protective blanket to prevent oxidation.

Endothermic Atmospheres: Active Chemical Processing
Endothermic atmospheres are highly reactive and are considered the workhorse for heat treatments that involve changing the surface properties of steel.
How It's Made: The Cracking Reaction
An endothermic atmosphere is produced in a generator where a precise, lean mixture of air and a hydrocarbon gas (like natural gas or methane) is passed over a heated catalyst, typically nickel.
This process is endothermic, meaning it consumes energy. The external heat is necessary to "crack" the hydrocarbon molecules, reforming them into the desired output gas.
Typical Composition and Its Role
The resulting gas mixture is a powerful agent for controlling metal surfaces. A standard composition is approximately:
- 40% Hydrogen (H₂): A strong reducing agent that actively removes oxygen, preventing scale and creating a bright surface finish.
- 20% Carbon Monoxide (CO): Provides the carbon potential necessary for carburizing or preventing decarburization in steels.
- 40% Nitrogen (N₂): Serves as an inert carrier gas, making up the balance of the atmosphere.
- Trace amounts of Carbon Dioxide (CO₂) and Water (H₂O): These are carefully controlled as they influence the gas's overall carbon potential.
Key Applications
The high H₂ and CO content makes endothermic gas ideal for processes where the surface metallurgy is critical.
- Bright Hardening: Hardening steel without creating surface oxides, resulting in a clean, bright part.
- Sintering: Bonding powdered metal particles together at high temperatures, requiring a reducing atmosphere to ensure proper fusion.
- Carbon Restoration: Reintroducing carbon into the surface of a steel part that was depleted during previous processing.
- Brazing: Joining metals with a filler material, where the reducing atmosphere ensures clean surfaces for a strong bond.
Exothermic Atmospheres: Protective Inerting
Exothermic atmospheres are generated through a simpler combustion process and are primarily used for protection rather than active surface treatment.
How It's Made: The Combustion Reaction
An exothermic atmosphere is created by burning a hydrocarbon gas with more air than in an endothermic generator. This process is exothermic, meaning it releases heat and is self-sustaining once ignited.
The level of combustion—rich or lean—determines the final composition and properties of the gas.
Rich vs. Lean: A Spectrum of Protection
There are two primary types of exothermic atmospheres.
- Rich Exothermic: Produced with partial combustion. It contains some reducing elements (~12% H₂, ~10% CO) but is less potent than endothermic gas. It's an excellent, low-cost protective blanket.
- Lean Exothermic: Produced with nearly complete combustion. It is mostly nitrogen (~87% N₂) with very low levels of H₂ and CO. It is largely inert but can be slightly oxidizing due to its higher CO₂ content.
Key Applications
Exothermic gas applications are chosen based on the level of protection needed.
- Rich Exo: Used for general-purpose annealing, tempering, and brazing of low-carbon steels where the risk of decarburization is minimal.
- Lean Exo: Primarily used for annealing non-ferrous metals like copper, where a highly reducing atmosphere is not required and slight oxidation is acceptable or even desired for surface finish.
Understanding the Trade-offs
Choosing the correct atmosphere requires understanding the direct consequences of their composition and generation method.
Reactivity and Process Control
Endothermic gas is highly reactive. Its carbon potential can be precisely controlled, making it essential for processes that modify the carbon content of a steel surface.
Exothermic gas is primarily protective. It prevents gross oxidation but has limited ability to control surface chemistry, with rich exo being mildly reducing and lean exo being nearly inert.
Cost and Complexity
Endothermic generators are more complex and costly. They require an external heat source, a catalyst bed that needs maintenance, and precise gas ratio controls to function correctly.
Exothermic generators are simpler, more robust, and less expensive to operate, as the reaction generates its own heat.
Safety Considerations
Both atmospheres contain flammable Hydrogen (H₂) and toxic Carbon Monoxide (CO). However, the significantly higher concentrations in endothermic gas (40% H₂, 20% CO) demand more stringent safety protocols, ventilation, and monitoring compared to exothermic atmospheres.
Making the Right Choice for Your Process
Your selection depends entirely on the metallurgical outcome you need to achieve.
- If your primary focus is adding carbon or actively preventing its loss (hardening, carburizing): An endothermic atmosphere is the only suitable choice due to its controllable carbon potential.
- If your primary focus is cost-effective oxidation prevention for non-critical steels: A rich exothermic atmosphere provides excellent protection for processes like general annealing or tempering.
- If your primary focus is treating non-ferrous metals like copper or requiring a mostly inert blanket: A lean exothermic atmosphere is the correct and most economical option.
Ultimately, understanding the fundamental chemical purpose of each gas empowers you to select the precise tool for your heat treatment application.
Summary Table:
| Aspect | Endothermic Atmosphere | Exothermic Atmosphere |
|---|---|---|
| Generation | Requires external heat; endothermic reaction | Self-sustaining heat release; exothermic reaction |
| Typical Composition | ~40% H₂, ~20% CO, ~40% N₂, trace CO₂/H₂O | Rich: ~12% H₂, ~10% CO, balance N₂; Lean: ~87% N₂, low H₂/CO |
| Primary Use | Active surface treatment (e.g., carburizing, bright hardening) | Protective atmosphere (e.g., annealing, oxidation prevention) |
| Cost & Complexity | Higher cost, more complex with catalyst and controls | Lower cost, simpler and robust operation |
| Safety | Higher H₂ and CO levels require strict protocols | Lower risk, but still needs ventilation and monitoring |
Need the right furnace for your atmosphere requirements? KINTEK specializes in advanced high-temperature solutions like Muffle, Tube, Rotary, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems. With strong R&D and deep customization, we tailor our products to meet your unique experimental needs—ensuring precise control and efficiency in heat treatment processes. Contact us today to discuss how we can enhance your lab's performance!
Visual Guide
Related Products
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
People Also Ask
- What is the use of nitrogen in furnace? Prevent Oxidation for Superior Heat Treatment
- What industries commonly use inert atmosphere heat treating? Key Applications in Military, Automotive, and More
- What are the two main types of atmosphere furnaces and their characteristics? Choose the Right Furnace for Your Lab
- Why is moisture control critical in inert atmosphere heat treating? Prevent Oxidation and Ensure Material Integrity
- What is the significance of nitrogen in atmosphere furnaces? Unlock Enhanced Heat Treatment and Surface Hardening



















