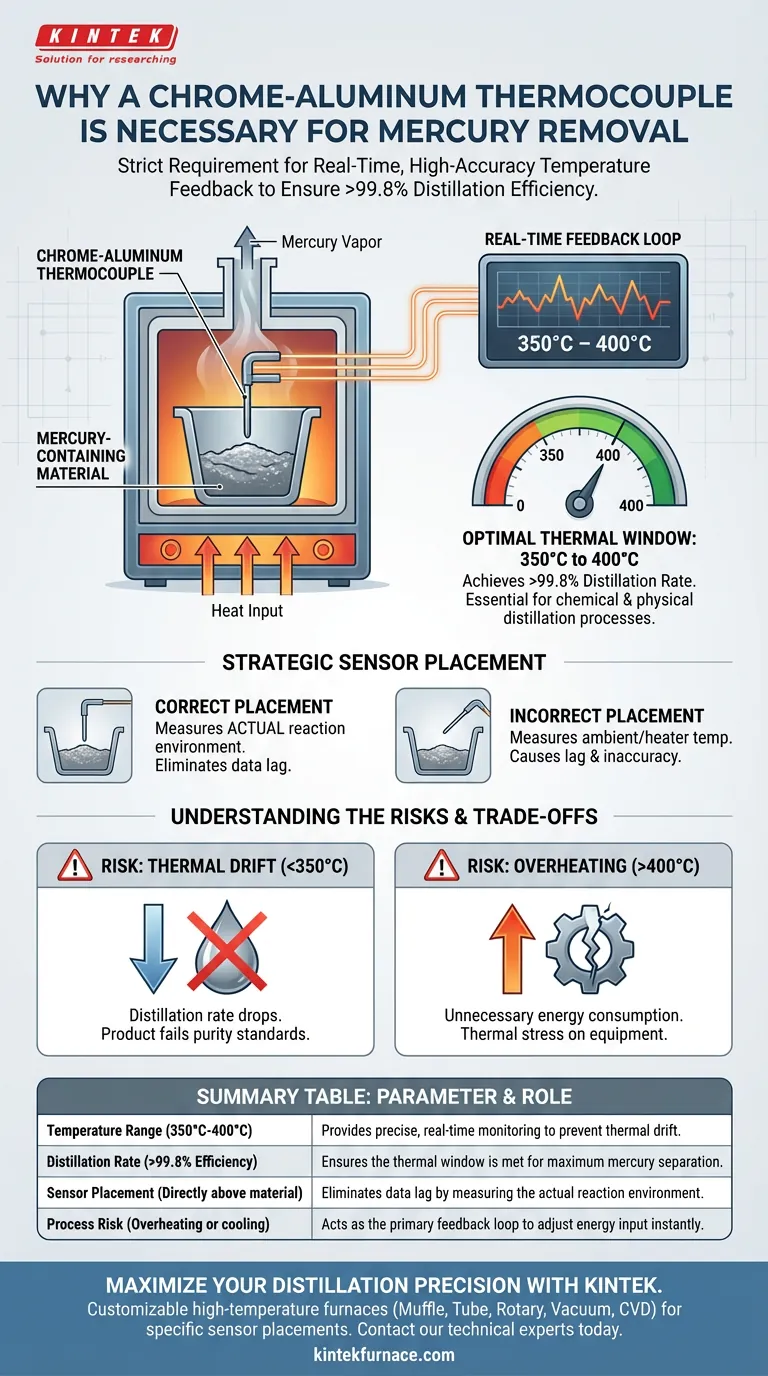
The necessity of a chrome-aluminum thermocouple in a mercury removal reaction space is driven by the strict requirement for real-time, high-accuracy temperature feedback. Because the efficiency of mercury distillation is volatile and dependent on specific thermal conditions, this sensor serves as the primary control mechanism to ensure the process remains within the narrow window required for success.
Mercury removal efficiency is highly sensitive to temperature, requiring a specific thermal environment to achieve distillation rates exceeding 99.8%. The chrome-aluminum thermocouple provides the precise, immediate data necessary to maintain the reaction space between the critical 350°C and 400°C thresholds.

The Critical Link Between Temperature and Efficiency
The Optimal Thermal Window
Mercury removal is not a process that benefits from "roughly" correct temperatures. The efficiency of the reaction reaches its peak only within a specific range: 350°C to 400°C.
Operating outside this band compromises the chemical and physical processes required for distillation. The chrome-aluminum thermocouple is essential because it offers the sensitivity required to hold the system within this 50-degree variance.
Achieving High Distillation Rates
The objective of this thermal treatment is to achieve a mercury distillation rate exceeding 99.8%.
This near-total removal is impossible without rigid thermal management. The thermocouple acts as the "eyes" of the system, verifying that the energy input is translating into the exact heat required to separate the mercury from the material.
Strategic Sensor Placement
Monitoring the Material, Not Just the Heater
For accurate control, the chrome-aluminum thermocouple is placed directly above the material in the reaction space.
This placement is deliberate. It ensures the system measures the actual temperature of the reacting material environment, rather than just the ambient heat of the chamber or the temperature of the heating elements.
Real-Time Feedback Loop
Thermal treatment processes can suffer from lag—the time between applying heat and the material reaching temperature.
By positioning the sensor close to the material, operators receive real-time feedback. This allows for immediate adjustments to energy input, preventing temperature spikes or drops that would disrupt the distillation process.
Understanding the Trade-offs
The Risk of Thermal Drift
Without the precise monitoring provided by a chrome-aluminum thermocouple, the system is prone to thermal drift.
If the temperature falls below 350°C, the distillation rate drops, leaving mercury behind in the material. This results in a product that fails to meet safety or purity standards.
Overheating and Efficiency Loss
Conversely, exceeding 400°C without accurate feedback can lead to unnecessary energy consumption.
While high heat ensures distillation, uncontrolled overheating puts thermal stress on the equipment and the reaction substrate without providing any additional benefit to mercury removal efficiency.
Making the Right Choice for Your Goal
To ensure your mercury removal system operates at peak performance, consider how you utilize temperature data.
- If your primary focus is Maximum Removal Efficiency: Prioritize maintaining the temperature strictly between 350°C and 400°C to consistently achieve >99.8% distillation rates.
- If your primary focus is System Responsiveness: Ensure the thermocouple is positioned directly above the material to eliminate data lag and allow for instant reaction to thermal changes.
Accurate sensing is the difference between a compliant, efficient process and a failed treatment cycle.
Summary Table:
| Parameter | Optimal Requirement | Role of Chrome-Aluminum Thermocouple |
|---|---|---|
| Temperature Range | 350°C to 400°C | Provides precise, real-time monitoring to prevent thermal drift. |
| Distillation Rate | > 99.8% Efficiency | Ensures the thermal window is met for maximum mercury separation. |
| Sensor Placement | Directly above material | Eliminates data lag by measuring the actual reaction environment. |
| Process Risk | Overheating or cooling | Acts as the primary feedback loop to adjust energy input instantly. |
Maximize Your Distillation Precision with KINTEK
Precise thermal control is the backbone of successful mercury removal and material purification. At KINTEK, we specialize in providing high-performance laboratory solutions backed by expert R&D and precision manufacturing. Whether you require Muffle, Tube, Rotary, Vacuum, or CVD systems, our high-temperature furnaces are fully customizable to accommodate specific sensor placements like chrome-aluminum thermocouples for your unique research needs.
Don't let thermal drift compromise your results. Contact our technical experts today to discover how KINTEK’s customizable thermal solutions can enhance your laboratory's efficiency and accuracy.
Visual Guide
References
- Bagdaulet Kenzhaliyev, Xeniya Linnik. Preliminary Removal of Mercury from Depleted Coal Sorbents by Thermal Vacuum Method with Associated Extraction of Precious Metal Composite. DOI: 10.3390/jcs8090367
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Molybdenum Disilicide MoSi2 Thermal Heating Elements for Electric Furnace
- Stainless Steel Quick Release Vacuum Chain Three Section Clamp
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
People Also Ask
- What are the typical applications of ceramic heating elements? Achieve Superior Heating for Your Industrial Processes
- What are the advantages of using MoSi2 heating elements in sintering furnaces? Boost Sintering Efficiency with Durable, Self-Healing Elements
- What are common failure modes of heating elements? Prevent Breakdowns and Extend Lifespan
- Why is platinum selected as a heating zone material for SEM furnaces? Ensuring High-Contrast Imaging at 1300°C
- What are the advantages of ceramic heating elements over metallic ones? Discover Superior Durability and Safety
- What are the key features of Silicon Carbide Heating Elements? Unlock High-Temp Precision and Durability
- What are the physical properties of molybdenum disilicide? Discover Its High-Temp Performance
- What are the advantages of using SiC heating elements in sintering furnaces? Boost Efficiency and Versatility




