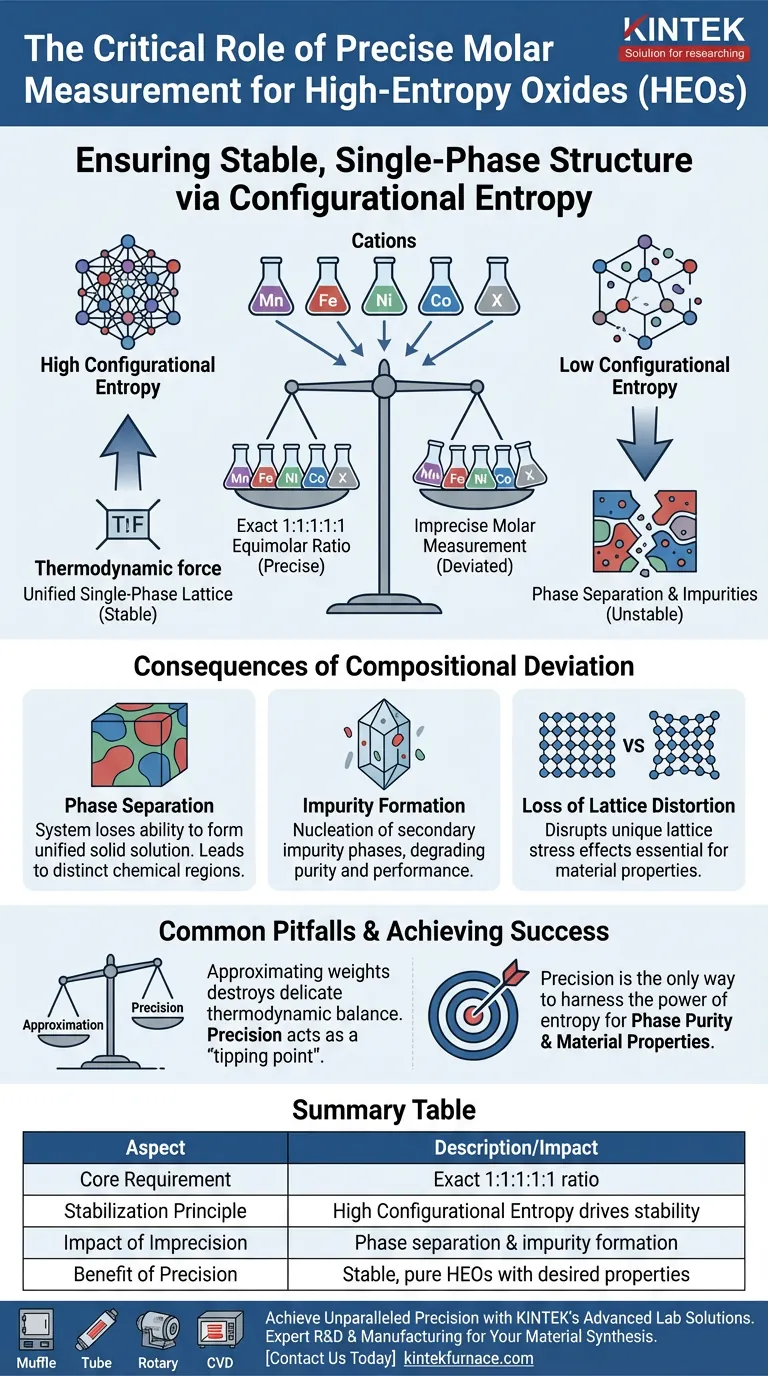
Precise molar measurement is strictly required to ensure that the five component metal elements (Mn, Fe, Ni, Co, and a fifth element X) achieve an exact equimolar ratio of 1:1:1:1:1. Without this specific stoichiometric balance, the material cannot generate the necessary configurational entropy to maintain a stable, single-phase structure.
Core Takeaway The stability of High-Entropy Oxides (HEOs) is not driven by standard chemical bonding preferences, but by configurational entropy. Inaccurate measurements lower this entropy, causing the unified crystal structure to collapse into unwanted secondary phases and impurities.
The Mechanism of Entropy Stabilization
The Equimolar Requirement
The fundamental definition of a high-entropy oxide relies on the simultaneous presence of five or more cations in equal proportions.
To maximize the "chaos" or entropy within the system, the molar ratio must be exactly 1:1:1:1:1.
Driving Structural Stability
Unlike traditional materials, HEOs are stabilized by high configurational entropy.
This entropy overcomes the enthalpy of mixing, effectively forcing distinct elements to coexist in a single crystal lattice.
If the measurement of the precursor metal nitrates is imprecise, the resulting cationic ratio shifts.
Consequently, the configurational entropy decreases, removing the thermodynamic force that holds the single-phase solid solution together.
Consequences of Compositional Deviation
Phase Separation
When the molar ratio deviates significantly, the system loses its ability to form a single-phase solid solution.
Instead of a unified material, phase separation occurs.
This results in a material containing distinct, chemically different regions rather than a homogenous lattice.
Formation of Impurities
Inaccurate measurements frequently lead to the nucleation of secondary impurity phases.
These impurities are thermodynamically more stable than the HEO structure when the entropy is insufficient to suppress them.
The presence of these secondary phases degrades the purity and performance of the final oxide.
Loss of Lattice Distortion
A key characteristic of HEOs is their unique lattice distortion effects, caused by the stress of fitting different-sized atoms into one structure.
Phase separation disrupts this distortion.
To preserve the unique physical properties derived from this lattice stress, the single-phase structure must be maintained through precise stoichiometry.
Common Pitfalls in Preparation
Underestimating Sensitivity
It is a common error to treat HEO precursors with the same tolerance levels as doped ceramics.
However, HEOs are far more sensitive; a lack of precision acts as a "tipping point."
The Risk of "Good Enough"
Approximating weights or volumes destroys the delicate thermodynamic balance of the synthesis.
Even minor deviations can shift the thermodynamics enough to favor the formation of simple binary oxides over the desired high-entropy phase.
Ensuring Synthesis Success
To achieve a high-quality high-entropy oxide, align your preparation technique with your specific goals:
- If your primary focus is Phase Purity: rigorous molar measurement is the only way to prevent phase separation and the formation of secondary impurities.
- If your primary focus is Material Properties: precision is required to maintain the specific lattice distortion effects that drive the material's unique behavior.
Precision in the lab is the only way to harness the thermodynamic power of entropy.
Summary Table:
| Aspect | Description/Impact |
|---|---|
| Core Requirement | Exact 1:1:1:1:1 equimolar ratio of constituent cations |
| Stabilization Principle | High Configurational Entropy drives single-phase stability |
| Impact of Imprecision | Lowers entropy, causes phase separation and impurity formation |
| Benefit of Precision | Ensures stable, pure HEOs with desired lattice distortion and properties |
Achieve unparalleled precision in your material synthesis experiments with KINTEK's advanced lab solutions. Backed by expert R&D and manufacturing, KINTEK offers Muffle, Tube, Rotary, Vacuum, CVD systems, and other lab high-temp furnaces, all customizable for unique needs. Ensure the success of your high-entropy oxide and other advanced material projects by leveraging our reliable and precise equipment. Contact us today to discuss how our tailored solutions can empower your research and manufacturing goals!
Visual Guide
References
- Milad Zehtab Salmasi, Hua Song. Tuning High-Entropy Oxides for Oxygen Evolution Reaction Through Electrocatalytic Water Splitting: Effects of (MnFeNiCoX)3O4 (X = Cr, Cu, Zn, and Cd) on Electrocatalytic Performance. DOI: 10.3390/catal15090827
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Magnesium Extraction and Purification Condensing Tube Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- Molybdenum Disilicide MoSi2 Thermal Heating Elements for Electric Furnace
- Chairside Dental Porcelain Zirconia Sintering Furnace with Transformer for Ceramic Restorations
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- What are the benefits of using a vacuum environment for RCM NSs? Master Material Preservation & Catalytic Performance
- What is the purpose of heating a precursor solution to 80 °C and 300 rpm stirring? Achieve High-Entropy Uniformity
- How does the structure of a shaft furnace facilitate the reduction of iron ore? Mastering High-Temp Heat Exchange
- What role do constant temperature water baths or ovens play in the sol-gel process for carbon aerogels? Master Kinetics
- How does a batch furnace differ from a continuous furnace? Choose the Right Furnace for Your Production Needs
- Importance of NaH2PO2 Layout in V-Ni3S2/NF Phosphorization: Ensuring Uniform 3D Doping
- How do vertical reaction furnaces simulate blast furnace reduction? Recover Iron from Steel Waste Effectively
- What is the primary function of adding bentonite and cement as binders? Optimize Iron Ore Briquette Strength




